Particles in charged solution form clusters that reproduce
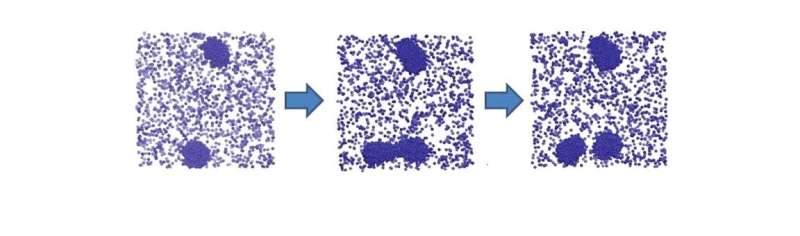
Dr Martin Sweatman from the University of Edinburgh's School of Engineering has discovered a simple physical principle that might explain how life started on Earth.
He has shown that particles that become charged in solution, like many biological molecules, can form giant clusters that can reproduce. Reproduction is shown to be driven by simple physics—a balance of forces between short-range attraction and long-range repulsion. Once cluster reproduction begins, he suggests chemical evolution of clusters could follow, leading eventually to life.
Many biological molecules, like DNA and proteins, might show this behaviour. Even the building blocks of life, amino acids and nucleobases, might show this behaviour. Reproduction in modern cells might even be driven by this simple physical mechanism, i.e. chemistry is not so important.
Dr Sweatman's research uses theoretical methods and computer simulations of simple particles. They clearly show giant clusters of molecules with the right balance of forces can reproduce. No chemistry is involved. However, these theoretical predictions have yet to be confirmed by experiment.
Dr Sweatman said, "Although it will be difficult to see this behaviour for solutions of small biomolecules, it should be possible to confirm this behaviour experimentally with much larger particles that can be seen under a microscope, like charged colloids.
"If this behaviour is confirmed, then we take another step towards Darwin's idea of life beginning in a warm little pond. A simple evaporation and condensation cycle in a pond might be sufficient to drive cluster reproduction initially. Survival of the fittest clusters of chemicals might then eventually lead to life."
The research has been published in the international journal Molecular Physics.
More information: M. B. Sweatman. Giant SALR cluster reproduction, with implications for their chemical evolution, Molecular Physics (2017). DOI: 10.1080/00268976.2017.1406164
Provided by University of Edinburgh