Scientists decipher mechanisms underlying the biology of aging

Understanding the factors that control aging has been one of humanity's endless pursuits, from the mystical fountain of youth to practical healthful regimens to prolong life expectancy.
A team of scientists at the University of California San Diego has helped decipher the dynamics that control how our cells age, and with it implications for extending human longevity. As described in a study published in the Proceedings of the National Academy of Sciences, a group led by biologist Nan Hao employed a combination of technologies in engineering, computer science and biology to analyze molecular processes that influence aging.
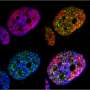
As cells age, damage in their DNA accumulates over time, leading to decay in normal functioning and eventually resulting in death. A natural biochemical process known as "chromatin silencing" helps protect DNA from damage. The silencing process converts specific regions of DNA from a loose, open state into a closed one, thus shielding DNA regions. Among the molecules that promote silencing is a family of proteins—broadly conserved from bacteria to humans—known as sirtuins. In recent years, chemical activators of sirtuins have received much attention and are being marketed as nutraceuticals to aid chromatin silencing in the hopes of slowing the aging process.
Yet at the same time, scientists have found that such chromatin silencing also stops the protected DNA regions from expressing RNAs and proteins that carry out biological functions, and as a result, excessive silencing could derail normal cell physiology.
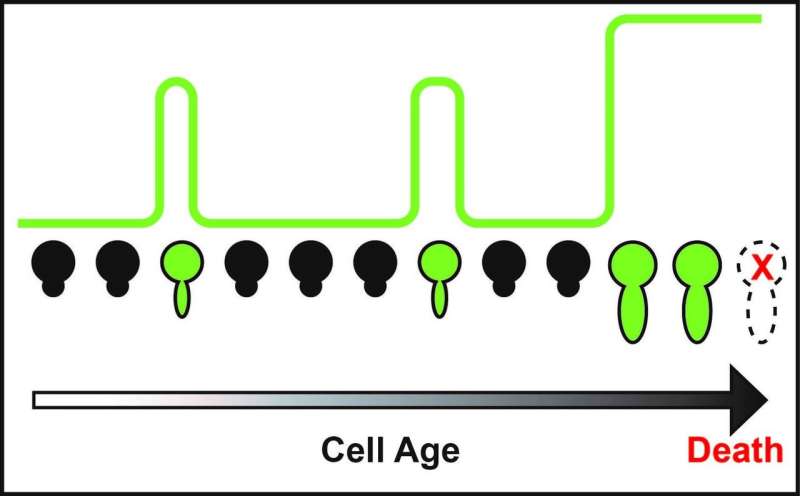
Using cutting-edge computational and experimental approaches in yeast, which allowed the researchers to track chromatin silencing in unprecedented detail through generations during aging, the UC San Diego scientists discovered that a complete loss of such silencing leads to accelerated cell aging and death. However, the researchers similarly found that continuous chromatin silencing also leads cells to a shortened lifespan.
So is chromatin silencing or not silencing the answer to delay aging? The answer derived from the new study: Both.
According to the researchers, nature has developed a clever way to solve this dilemma.
"Instead of staying in the silencing or silencing loss state, cells switch their DNA between the open (silencing loss) and closed (silencing) states periodically during aging," said Hao. "In this way, cells can avoid a prolonged duration in either state, which is detrimental, and maintain a time-based balance important for their function and longevity."
Because they conducted their experiments in yeast, the researchers say analyzing such dynamics in humans is likely to be much more complex and require more intricate studies.
"When cells grow old, they lose their ability to maintain this periodic switching, resulting in aged phenotypes and eventually death," said Hao. "The implication here is that if we can somehow help cells to reinforce switching, especially as they age, we can slow their aging. And this possibility is what we are currently pursuing."
Hao credits the findings to a multidisciplinary team of UC San Diego researchers who have complementary expertise and share a common interest in the study of aging, including faculty, students and postdoctoral fellows. In addition to Hao's expertise in Molecular Biology/Quantitative Biology, other faculty members in the team include Lorraine Pillus (Molecular Biology), Jeff Hasty (Molecular Biology/Bioengineering) and Lev Tsimring (BioCircuits Institute). The PNAS paper marks the first significant findings from the collaboration.
"I believe this collaboration will produce in the near future many new insights that will transform our understanding in the basic biology of aging and will lead to new strategies to promote longevity in humans," said Hao.
More information: Yang Li et al. Multigenerational silencing dynamics control cell aging, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1703379114
Journal information: Proceedings of the National Academy of Sciences
Provided by University of California - San Diego