The electronic origins of fluorescence in carbon nanotubes
Technological progress is often driven by materials science. High-tech devices require "smart" materials that combine a range of properties. An impressive current example is carbon nanotubes (CNTs)—single sheets of carbon atoms rolled into a cylinder. These ultrathin tubes have enormous mechanical strength and electrical conductivity. They also emit infrared fluorescent light, rendering them detectable. This makes them exciting materials for future bio-imaging technology, but the mechanism has proven surprisingly elusive.
The frequency of infrared light emitted by CNTs is shifted when organic molecules are attached to the outside of the tubes. This provides a way to "tune" the fluorescence depending on the required purpose. However, the origin of the frequency shift is hard to investigate, because only a few molecules are actually placed on the tubes. Standard methods therefore struggle to pinpoint them— a needle-in-a-haystack type of task.
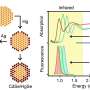
Now, a trio of researchers at Japan's Kyushu University has made progress in understanding these frequency shifts at the atomic level. In a study published in Nanoscale, they report using the technique of spectro-electrochemistry—applying an electrical potential ("electro") to a fluorescent material, and measuring the resulting emission of light ("spectro"). The use of electricity reveals the electron energy levels in the CNTs—i.e., the orbitals around atoms. This is crucial, because fluorescence consists of "excited" electrons moving from one orbital to another, then releasing energy in the form of light.
"The frequency of fluorescence from CNTs depends on the gaps between electron energy levels," the lead authors explain. "These gaps in turn depend on which elements are bonded to the exterior of the nanotubes. For example, we found that molecules containing bromine pushed the energy levels closer together compared to molecules with hydrogen at the same position."
This narrows the gap—mostly by raising the highest occupied orbital, bringing it closer to the empty orbitals above it—and results in fluorescence with a lower frequency.
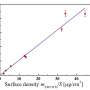
The measured changes in electronic states were consistent with the fluorescent shifts. This confirmed that the electron energy levels were the key to frequency tuning, allowing the researchers to rule out an alternative explanation based on the stability of excited electrons. It seems that the effect is mainly caused by the electric field, or dipole, that is generated when molecules are bonded to the CNTs. This field, in turn, depends on the ability of those molecules to pull electrons away from the carbon in the nanotubes.
"Fluorescent CNTs could play a huge role in biomedicine," the authors say. "Our study method, based on electrochemistry, will allow researchers to understand fluorescent materials in full electronic detail. In the near future, this will open the way to fine-tuning of CNTs, in terms of both optical frequency and brightness, by carefully directed chemical decoration."
The article, "Substituent effects on the redox states of locally functionalized single-walled carbon nanotubes revealed by in situ photoluminescence spectroelectrochemistry," was published in Nanoscale.
More information: Tomonari Shiraishi et al, Substituent effects on the redox states of locally functionalized single-walled carbon nanotubes revealed by in situ photoluminescence spectroelectrochemistry, Nanoscale (2017). DOI: 10.1039/c7nr05480g
Journal information: Nanoscale
Provided by Kyushu University