Hollow atoms: The consequences of an underestimated effect
The "hollow atoms", which are being produced in the labs of TU Wien (Vienna) are quite exotic objects. Their electrons are in a state of extremely high energy (so called Rydberg states), but when they are shot through another material, they can get rid of this energy in a matter of femtoseconds (millionths of a billionth of a second).
For a long time, physicists have been speculating how this process can be so fast. Experiments with xenon ions and graphene have now shown that the reason is an effect which has been hugely underestimated: the so-called "interatomic coulomb decay". Studying this effect is not only important for atomic physics, but also for our health: when biological material is irradiated, the interatomic coulomb decay can fracture DNA molecules. These results have now been published in the journal Physical Review Letters.
Hollow Atoms
Extreme environments are created in the labs at TU Wien. In an ion trap, large amounts of energy are used to rip a great number of electrons out of their atoms, leaving highly charged ions behind. When such an ion is fired onto a surface, it regains its electrons, pulling them away from the surface. These new electrons, however, have very high energies. They occupy the outer electron shells, far away from the atomic nucleus - whereas in a normal atom, the electrons tend to occupy the innermost electron shells, where their energy is low. An atom, in which many electrons are located in the outer electron shells while many inner electron states are empty, is called a "hollow atom".
"As soon as these hollow atoms enter a solid, for example, when they penetrate a thin foil, their electronic state changes almost instantaneously", says Richard Wilhelm, a scientist in Prof. Friedrich Aumayr's team at TU Wien. "The highly excited electrons return to a state of lower energy. And this happens so fast that for many years it remained a mystery, which process can be responsible for that energy transfer."
"The usual mechanisms which normally allow electrons to get rid of their energy are much too slow", says Friedrich Aumayr. "Therefore, different ad-hoc-hypotheses have been proposed to explain this phenomenon. But nobody really had a satisfying answer."
Xenon and Graphene
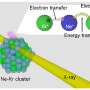
Together with physicists from the Helmholtz-Zentrum Dresden-Rossendorf, the Viennese team decided to take a closer look. They used very heavy ions - thirty-fold positively charged xenon atoms - and fired them onto graphene, the world's thinnest material, consisting of only one layer of carbon atoms. The time it takes the charged atoms to traverse the graphene is just one femtosecond, but this ultrashort contact is enough to completely change the distribution of electrons.
The experiment showed that this redistribution is due to an effect, which has been considered to be rather unimportant - the interatomic Coulomb decay: the energy of a single electron is transferred to several other electrons of neighbouring atoms. The highly charged xenon atom passes through the graphene layer and comes into contact with several carbon atoms at the same time. The high energy of an electron in the xenon atom is passed on to several electrons in the graphene which can now leave their place and hurtle away - but only with rather low energies.
The low energy of the resulting electrons is the reason why this process plays an interesting part in biology. Such interatomic coulomb decays can also happen when ionizing radiation (as it is used in cancer therapy, when patients are irradiated with gamma radiation, ions or electrons) removes an inner electron from an atom and leaves the atom in a highly excited ("hollow") state. Also in that case, the energy can be distributed over several neighbouring atoms, and many slow electrons are emitted. This can lead to single- or double-strand breaks in DNA molecules. In normal human tissue, this can cause inherited defects or cancer, but in radiation therapy, this kind of DNA damage can be very effective in destroying cancer cells.
These new insights about the important role of the interatomic coulomb decay in hollow atoms open up new ways of studying this effect and gaining new insights which are relevant for medicine and biology.
More information: Richard A. Wilhelm et al, Interatomic Coulombic Decay: The Mechanism for Rapid Deexcitation of Hollow Atoms, Physical Review Letters (2017). DOI: 10.1103/PhysRevLett.119.103401
Journal information: Physical Review Letters
Provided by Vienna University of Technology