Interactive protein posttranslational modifications regulate stress responses
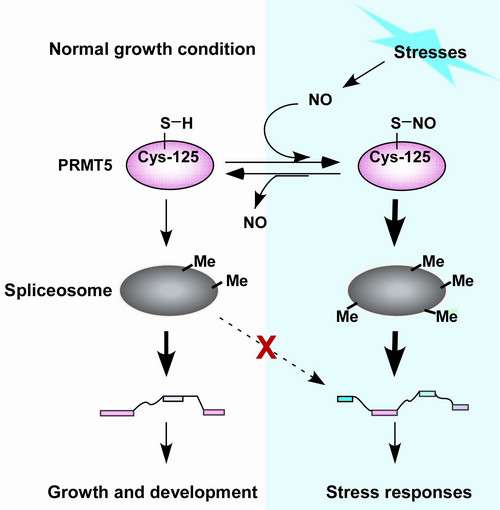
Methylation and nitric oxide (NO)-based S-nitrosylation are highly conserved protein posttranslational modifications that regulate diverse biological processes, including abiotic stress responses. However, little is known about the underlying molecular mechanisms.
Dr. ZUO Jianru's group at the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences identified PRMT5, a protein arginine methyltransferase, as an S-nitrosylated protein in a nitroproteomics study in Arabidopsis. PRMT5 is a highly conserved enzyme that catalyzes arginine symmetric dimethylation of various proteins, including key components of the spliceosome.
With collaborators, the researchers discovered that NO positively regulates the methyltransferase activity of PRMT5 through S-nitrosylation at Cys-125 in response to abiotic stresses. Consequently, S-nitrosylation at Cys-125 of PRMT5 enhances arginine symmetric dimethylation, leading to proper splicing-specific pre-mRNA of stress-related genes and eventually boosting the tolerance to stress. Importantly, S-nitrosylation at Cys-125 is essential for PRMT5-regulated stress responses mediated by NO.
These findings define a molecular link through which plants transduce stress-triggered NO signals to protein methylation machinery in response to environmental alterations. The results of this research, entitled "Nitric oxide regulates protein methylation during stress responses in plants", were recently published online in Molecular Cell.
More information: Jiliang Hu et al, Nitric Oxide Regulates Protein Methylation during Stress Responses in Plants, Molecular Cell (2017). DOI: 10.1016/j.molcel.2017.06.031
Journal information: Molecular Cell
Provided by Chinese Academy of Sciences