Formation of porous crystals observed for the first time
Scientists at the University of Bristol have, for the first time, observed the formation of a crystal gel with particle-level resolution, allowing them to study the conditions by which these new materials form.
The study showed that the mechanism of crystal growth follows the same strategies by which ice crystals grow in clouds, an analogy which could improve our understanding of these fundamental processes
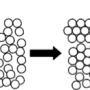
In addition, this novel mechanism allowed the research team to spontaneously form sponge-like nanoporous crystals in a continuous process.
Nanoporous crystals of metals and semiconductors can be obtained without dealloying, which can be important for catalytic, optical, sensing, and filtration applications.
The work is a collaboration between the University of Tokyo (where the experiments were conducted), Bristol and the Institute Lumiere Matiere in Lyon, France.
The findings are published today in the journal, Nature Materials.
Dr John Russo, from the University of Bristol's School of Mathematics and co-author of the research paper, said: "In particular we observed some new formation mechanisms.
"We discovered that in order to obtain these crystal-gel structures, the original gel structure has to undergo a structural reorganisation, in which bonds between colloidal particles are broken to release the internal stress that was accumulated during the rapid growth of the gel - a process called stress driven aging.
"After this, we observed that the way the branches of the gel crystallise is reminiscent of the process by which water droplets crystallise in clouds. We were then able to observe processes that promote crystallisation through an intermediate gas phase.
"This is the first time these fundamental processes are observed at a particle-level resolution, which gives us unprecedented insight over how the process occurs."
The paper reports on experiments on an out-of-equilibrium phase of matter which is obtained by mixing colloidal particles of micronmeter size, with short polymer chains in a good solvent.
The role of the polymers is to induce an effective attraction between the colloidal particles, due to a physical effect called depletion, whose origin is purely entropic.
At the beginning of the experiment, colloidal particles repel each other due to electrostatic repulsion. In order to induce the depletion attraction between colloids, the sample is put in contact with a salt solution through a semi-permeable membrane.
As the salt diffuses through the semi-permeable membrane, it screens the electrostatic repulsion between the colloidal particles, which then start to aggregate.
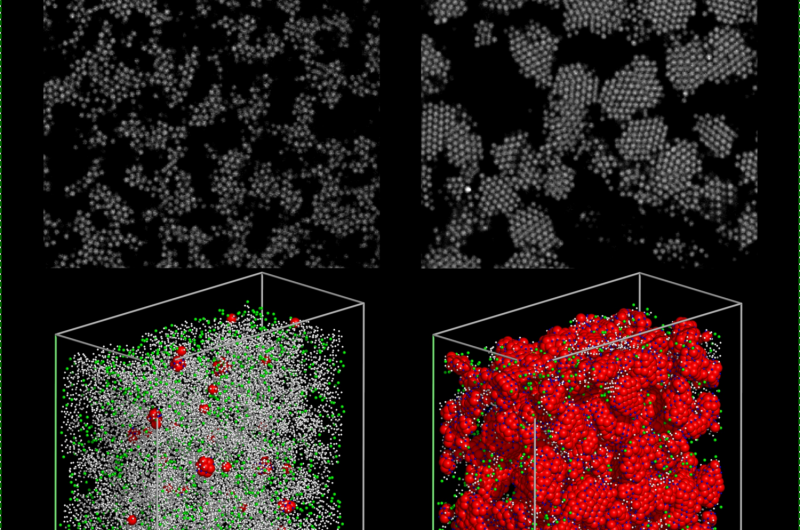
The whole process of aggregation is observed with a confocal microscope, which takes fast scans of the sample at different heights, so that the researchers can reconstruct the coordinates of the colloidal particles with image analysis, and study how these particles move over the course of several hours.
If the polymer concentration is high, the system will form a gel - a disordered state in which colloidal particles aggregate to form interconnected branches that span the whole system, and that give rigidity to the structure.
Dr Russo added: "What we have demonstrated, instead, is that if we tune the polymer concentration at right value (next to what is called a critical point), the system will not form a different type of gel, in which the colloidal particles crystallise throughout the gel structure, giving origin to a porous material made of crystalline branches."
More information: Formation of porous crystals via viscoelastic phase separation, Nature Materials (2017). DOI: 10.1038/nmat4945
Journal information: Nature Materials
Provided by University of Bristol