February 23, 2017 feature
Scientists solve puzzle of turning graphite into diamond
(Phys.org)—Researchers have finally answered a question that has eluded scientists for years: when exposed to moderately high pressures, why does graphite turn into hexagonal diamond (also called lonsdaleite) and not the more familiar cubic diamond, as predicted by theory?
The answer largely comes down to a matter of speed—or in chemistry terms, the reaction kinetics. Using a brand new type of simulation, the researchers identified the lowest-energy pathways in the graphite-to-diamond transition and found that the transition to hexagonal diamond is about 40 times faster than the transition to cubic diamond. Even when cubic diamond does begin to form, a large amount of hexagonal diamond is still mixed in.
The researchers, Yao-Ping Xie, Xiao-Jie Zhang, and Zhi-Pan Liu at Fudan University and Shanghai University in Shanghai, China, have published their study on the new simulations of the graphite-to-diamond transition in a recent issue of the Journal of the American Chemical Society.
"This work resolves the long-standing puzzle of why hexagonal diamond is preferentially produced from graphite instead of the cubic diamond at the onset of diamond formation," Liu told Phys.org. "Considering that graphite-to-diamond is a prototype solid-to-solid transition, the knowledge learned from this work should greatly benefit the understanding of high-pressure solid physics and chemistry."
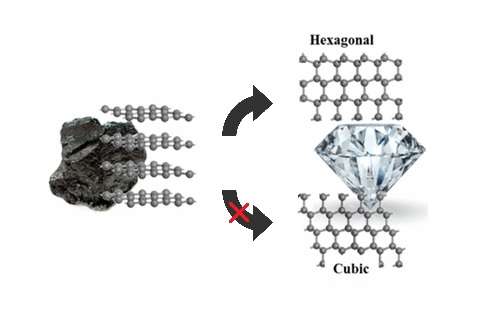
Graphite, hexagonal diamond, and cubic diamond are all carbon allotropes, meaning they are made of carbon atoms that are arranged in different ways. Graphite consists of stacked layers of graphene, whose atoms are arranged in a honeycomb-like lattice. Since the carbon atoms in graphene are not fully bonded, graphene is soft and flakes easily, making it ideal for use as pencil lead.
Both types of diamond, on the other hand, consist of carbon atoms that all have the maximum four bonds, which explains why diamond is so hard. In cubic diamond (the kind typically found in jewelry), the layers are all oriented in the same direction. In hexagonal diamond, the layers are alternately oriented, giving it a hexagonal symmetry.
Under high pressures of more than 20 gigapascals (nearly 200,000 times atmospheric pressure), theory and experiment agree that graphite turns into cubic diamond, with some hexagonal diamond mixed in. But under pressures of less than 20 gigapascals, simulations have always predicted that cubic diamond should be the favored product, in contrast with experiments.
These simulations are based on the prediction that, at these pressures, less energy is required to form the cubic diamond nucleation core, or nucleus—the starting point of diamond growth—than to form the hexagonal diamond nucleus. Since forming this nucleus is the most energy-consuming step of the entire process, it follows that cubic diamond formation should be more thermodynamically favorable than hexagonal diamond.
But a major drawback of these simulations is that they do not account for the interfaces between the graphite and the diamond nuclei: a lattice mismatch between the two surfaces can induce a strain energy that can interfere with the stability of the growing diamond.
Using a novel simulation called stochastic surface walking, the researchers in the new study could more thoroughly explore all of the possible interfaces and identify seven of them that correspond to the lowest-energy intermediate structures in the graphite-to-diamond transition.
Overall, the results show that the interface between graphite and the hexagonal diamond nucleus is less strained and more stable than the interface with the cubic diamond nucleus. Accounting for the stability of these interfaces can finally explain why hexagonal diamond forms much more easily and quickly than cubic diamond at moderate pressures.
The researchers added that, although cubic diamond may appear to be more desirable than hexagonal diamond to the average person, both materials have their advantages.
"While cubic diamond is familiar in everyday life and is a highly useful material, hexagonal diamond could also be very useful," Liu said. "For example, it was predicted by theory to be even harder than cubic diamond. While the hexagonal diamond (lonsdaleite) can be found in meteorites, the production of large hexagonal diamond crystals has not been achieved in experiment. One would therefore expect that large hexagonal diamond crystals, if produced, would be even more precious than cubic diamond."
In the future, the researchers are planning to further improve the simulations by incorporating techniques from neural networks as well as by using big data.
More information: Yao-Ping Xie et al. "Graphite to Diamond: Origin for Kinetics Selectivity." Journal of the American Chemical Society. DOI: 10.1021/jacs.6b11193
Journal information: Journal of the American Chemical Society
© 2017 Phys.org