New technique switches key biomolecules on and off
A new technique that will allow scientists to determine the effects of turning on and off a set of molecules involved in almost every cellular pathway, determine their downstream effects, and uncover new drug targets has been developed by researchers at the University of Illinois at Chicago.
The finding is reported in the Proceedings of the National Academy of Sciences.
Protein kinases are enzymes involved in almost every biological process. These molecules perform a single action - they transfer phosphate from the energy molecule ATP to other enzymes and proteins. The process is a key mechanism of regulatory control and allows cells to perform all of their functions.
Several hundred different kinases work to phosphorylate various proteins and drive the entire range of cellular activities. Understanding precisely how kinases work would reveal cellular pathways that drive cells to become cancerous and identify novel drug targets.
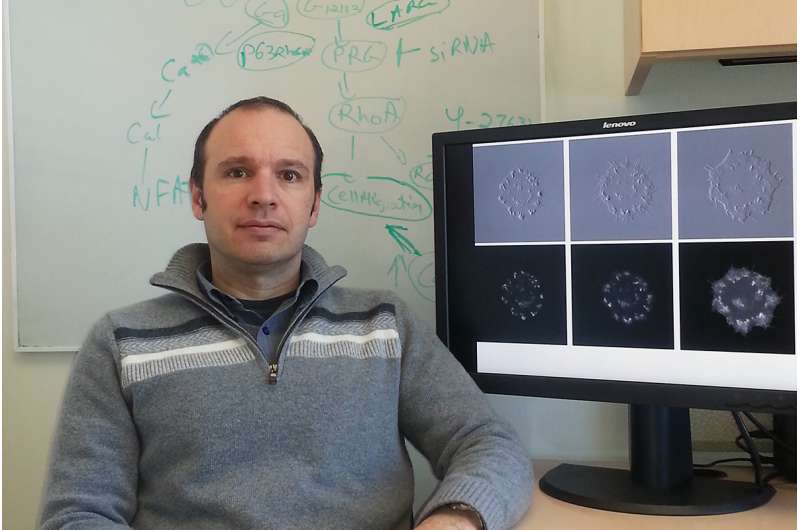
Until now, it was difficult to study cellular processes triggered by specific kinases that turn on only briefly, says Andrei Karginov, assistant professor of pharmacology in the UIC College of Medicine.
"Previously, we had the ability to artificially turn on a kinase, but we didn't know how to turn it off," Karginov said. "So what we could see was only the result of that kinase being on for a long time."
That approach has proved useful to study the prolonged cancer-causing effects of some kinases, Karginov said.
"But, in living cells, kinases are often turned only for a set period of time, and the duration of this activation often dictates what will happen to the cell," he said.
"To mimic this transient action we had come up with a new strategy."
The new procedure allows scientists to turn kinases on, and then off - providing a powerful tool for studying the effects of kinases as they actually work in the body.
The researchers used two protein-engineering techniques to switch on and switch off one kinase, called tyrosine kinase c-Src. They were able to observe a series of structural changes when they turned the enzyme on for various periods of time.
The first wave of changes, where the cell seemed to expand and then contract, was driven by kinase activation and inactivation. Interestingly, a second expansion sometimes occurred after the kinase had been switched off - depending on how long it had been left on.
The experiment demonstrated that even short-term activation of a kinase can have a long-lasting effect on cell behavior, and allowed researchers to identify the molecular mechanism driving these events.
More information: Jennifer E. Klomp et al. Mimicking transient activation of protein kinases in living cells, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1609675114
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Illinois at Chicago