Solar cells get boost with integration of water-splitting catalyst onto semiconductor
Scientists have found a way to engineer the atomic-scale chemical properties of a water-splitting catalyst for integration with a solar cell, and the result is a big boost to the stability and efficiency of artificial photosynthesis.
Led by researchers at the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab), the project is described in a paper published this week in the journal Nature Materials.
The research comes out of the Joint Center for Artificial Photosynthesis (JCAP), a DOE Energy Innovation Hub established in 2010 to develop a cost-effective method of turning sunlight, water, and carbon dioxide into fuel. JCAP is led by the California Institute of Technology with Berkeley Lab as a major partner.
The goal of this study was to strike a careful balance between the contradictory needs for efficient energy conversion and chemically sensitive electronic components to develop a viable system of artificial photosynthesis to generate clean fuel.
Striking the right balance
"In order for an artificial photosystem to be viable, we need to be able to make it once, deploy it, and have it last for 20 or more years without repairing it," said study principal investigator Ian Sharp, head of materials integration and interface science research at JCAP.
The problem is that the active chemical environments needed for artificial photosynthesis are damaging to the semiconductors used to capture solar energy and power the device.
"Good protection layers are dense and chemically inactive. That is completely at odds with the characteristics of an efficient catalyst, which helps to split water to store the energy of light in chemical bonds," said Sharp, who is also a staff scientist at Berkeley Lab's Chemical Sciences Division. "The most efficient catalysts tend to be permeable and easily transform from one phase to another. These types of materials would usually be considered poor choices for protecting electronic components."
By engineering an atomically precise film so that it can support chemical reactions without damaging sensitive semiconductors, the researchers managed to satisfy contradictory needs for artificial photosystems.
"This gets into the key aspects of our work," said study lead author Jinhui Yang, who conducted the work as a postdoctoral researcher at JCAP. "We set out to turn the catalyst into a protective coating that balances these competing properties."

Doing double duty
The researchers knew they needed a catalyst that could not only support active and efficient chemical reactions, but one that could also provide a stable interface with the semiconductor, allow the charge generated by the absorption of light from the semiconductor to be efficiently transferred to the sites doing catalysis, and permit as much light as possible to pass through.
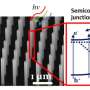
They turned to a manufacturing technique called plasma-enhanced atomic layer deposition, performed at the Molecular Foundry at Berkeley Lab. This type of thin-film deposition is used in the semiconductor industry to manufacture integrated circuits.
"This technique gave us the level of precision we needed to create the composite film," said Yang. "We were able to engineer a very thin layer to protect the sensitive semiconductor, then atomically join another active layer to carry out the catalytic reactions, all in a single process."
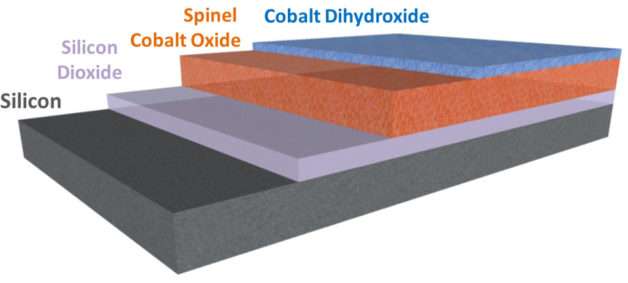
The first layer of the film consisted of a nanocrystalline form of cobalt oxide that provided a stable, physically robust interface with the light-absorbing semiconductor. The other layer was a chemically reactive material made of cobalt dihydroxide.
"The design of this composite coating was inspired by recent advances in the field that have revealed how water-splitting reactions occur, at the atomic scale, on materials. In this way, mechanistic insights guide how to make systems that have the functional properties we need," said Sharp.
Using this configuration, the researchers could run photosystems continuously for three days—potentially longer—when such systems would normally fail in mere seconds.
"A major impact of this work is to demonstrate the value of designing catalysts for integration with semiconductors," said Yang. "Using a combination of spectroscopic and electrochemical methods, we showed that these films can be made compact and continuous at the nanometer scale, thus minimizing parasitic light absorption when integrated on top of photoactive semiconductors."
The study authors noted that while this is an important milestone, there are many more steps needed before a commercially viable artificial photosystem is ready for deployment.
"In general, we need to know more about how these systems fail so we can identify areas to target for future improvement," said Sharp. "Understanding degradation is an important avenue to making something that is stable for decades."
More information: Jinhui Yang et al. A multifunctional biphasic water splitting catalyst tailored for integration with high-performance semiconductor photoanodes, Nature Materials (2016). DOI: 10.1038/nmat4794
Journal information: Nature Materials
Provided by Lawrence Berkeley National Laboratory