November 14, 2016 report
Checkmate for Castleman disease
(Phys.org)—Dr. David Fajgenbaum is the founder of Castleman Disease Collaborative Network. Its goal is to organize patients with Castleman disease (CD), find an explanation for this rare and enigmatic immunological disorder that involves the immune system attacking vital organs, and beat it. As an orphan disease researcher at Penn, and a sufferer of CD himself, David's first-person, n-of-1 approach to a medical brick wall has emerged as the new, and only viable blueprint for moving the bar forward in real time.
In order to make progress for rare diseases, David tells me, he realized that if he didn't drive things forward than no one would. After being given his diagnosis as a medical student concentrating on oncology, David switched gears and made CD his first order of business. When he observed all the hurdles in the way of progress, he decided to get an MBA at Wharton, developing business contacts and funding sources for the network of CD researchers he was building. He created a community for patients and physicians to collaborate and share high-potential research ideas. In parallel, he began to conduct CD research at Penn and identify top experts to conduct other priority projects. More recently, the CDCN launched a registry of people with CD so they could share patient records and unpublished cases of the disease.
An immediate consequence of these efforts was a redefinition of the more deadly form of CD disease—the so-called 'multicentric' form that involves several regions of enlarged lymph nodes as opposed to just one. The former consensus held that benign lymph node tumors secreted Interleukin-6 (IL-6), which in turn hyperactivated the immune system and caused widespread organ dysfunction. The new explanation is pretty much the opposite. In other words, an overactive immune system is what seems to cause the tumors, flu-like symptoms and organ system dysfunction in the first place. The job is therefore to first explain the cause of hyperactivation of different proinflammatory cells and proteins (including Il-6), and then identify ways to stop them.
In this view, multicentric CD can be seen as a common endpoint that can be expressed by traversing different paths. In some cases it is due to infection with the HHV-8 virus. This particular pathogen happens to express its own Il-6 type protein that, despite having just 40% sequence homology with our own Il-6, still manages to cause havoc. HHV-8 causes about half of the multicentric form of CD and provides one of many important clues. Other cases, which are called "idiopathic multicentric CD", may be due as yet unknown viral infection, purely autoimmune mechanisms, or even germ-line disturbances in innate immune regulation. One thing that could be a complication involved in the latter is the increasingly common finding of individuals possessing mosaic and chimeric germ lines.
Genetic sequencing and subsequent functional proteomics will be one valuable source of new information here. Once suspect genes are identified they can potentially be expressed in different cells to see what effects different mutants may have on function. These days, it is perhaps just as likely that advanced simulation tools based on molecular dynamics will give the first functional clues about changes in protein folding or function in the cell.
David mentioned that 15 patients, himself included are now getting whole genome sequencing done. This is much more useful than the commonly done 'exome sequencing' which can miss many important details including polymorphism in the promoter and other regulatory regions that get spliced out before the exomes are generated. Other critical aberrants like gene duplications will generally be missed in exome sequencing as well.
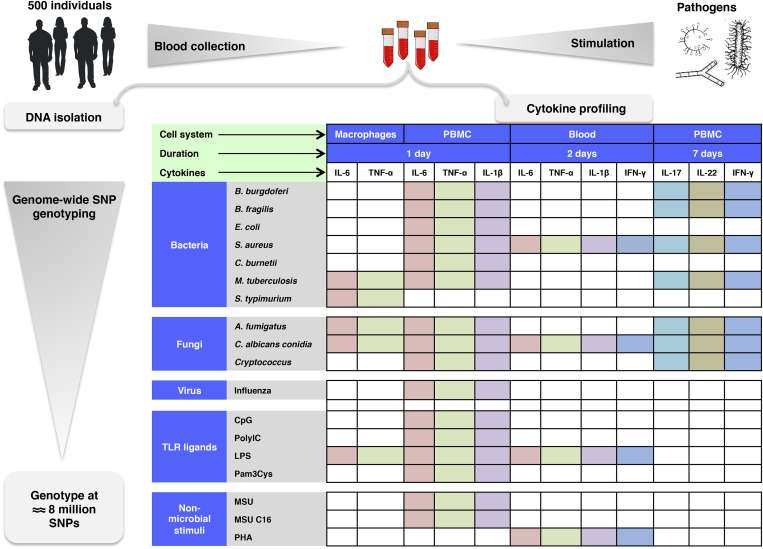
A recent series of papers in Cell takes a functional genomics approach to understand variation in human cytokine production. After challenging cells from hundreds of different people with various bacterial, fungal, viral, and non-microbial stimuli, researchers comprehensively identified the cytokine 'quantitative trait loci' that influence the major cytokines (Il-6 included) in whole blood, in blood mononuclear cells, and in macrophages. These studies also looked at other different host and environmental factors, like for example, the influence of the gut microbiome. If reformulated more directly to CD patients important information at the individual scale may be obtained. Among other things, this could include better ways to take critical measurements. For example, developing a more uniform sampling protocol that takes into account the known daily, seasonal, and other natural rhythms of the body's many organ and systems.
David is just one of many researchers at Penn, and elsewhere, who are now taking matters into their own hands. I am reminded of Scott Mackler, a former Penn researcher I consulted for some time ago. When diagnosed with ALS, Scott repurposed his labs efforts to focus on his this devastating disease. In checking now, Scott passed back in 2013 after a 15 year battle, but his legacy lives on. Today, several other home grown efforts are achieving incredible results. In one notable case, a mother with no previous medical background, and no initial funding, was able to found a company called Lysogene that in just a few years developed an FDA approved genetic therapy for the Sanfilippo disease that affected her newborn.
Another instance is the case of Shirley Pepke, a genomics researcher who developed machine learning tools to tailor treatments for combating her ovarian cancer. In remission now, Shirley took what seemed like a big chance in going against her oncologist's recommendation of standard chemotherapy to try a new (an non-FDA approved) immuntherapy drug. Although it may not be possible to fully disambiguate exactly which of the Shirleys many different treatments 'worked', the important thing for her is that she is in fact now in remission. This general philosophy is in stark contrast to the 'drug trial centric' approach where the study is the goal rather than the individual.
Perhaps an even more high profile case is that of Tom Marsilje, a research at Novartis who codeveloped an anti-cancer ALK inhibitor now marketed as Zykadia. Tom needed to get into a trial to treat his own colon cancer but was triaged out of it because he happened to have a pre-existing melanoma. A somewhat fortuitous but perhaps inevitable circumstance brought Tom together with Craig Venter who was promptly able to whole genome sequence Tom's cells. Using the data rendered they are now developing a personalized neoantigen vaccine, an immunotherapy, that may be able to stimulate his own immune cells to attack tumor.
In another fortunate turn of events, the Castlemen network is pleased to be partnering with Janssen Research and Development, and Penn, to create the first global patient registry for CD. Fajgenbaum is serving as principle investigator on this new initiative to help defeat Castlemen disease once and for all.
More information: Yang Li et al. A Functional Genomics Approach to Understand Variation in Cytokine Production in Humans, Cell (2016). DOI: 10.1016/j.cell.2016.10.017
Abstract
As part of the Human Functional Genomics Project, which aims to understand the factors that determine the variability of immune responses, we investigated genetic variants affecting cytokine production in response to ex vivo stimulation in two independent cohorts of 500 and 200 healthy individuals. We demonstrate a strong impact of genetic heritability on cytokine production capacity after challenge with bacterial, fungal, viral, and non-microbial stimuli. In addition to 17 novel genome-wide significant cytokine QTLs (cQTLs), our study provides a comprehensive picture of the genetic variants that influence six different cytokines in whole blood, blood mononuclear cells, and macrophages. Important biological pathways that contain cytokine QTLs map to pattern recognition receptors (TLR1-6-10 cluster), cytokine and complement inhibitors, and the kallikrein system. The cytokine QTLs show enrichment for monocyte-specific enhancers, are more often located in regions under positive selection, and are significantly enriched among SNPs associated with infections and immune-mediated diseases.
Journal information: Cell
© 2016 Phys.org