July 26, 2016 report
Synthesis and characterization of encapsulated single HF molecule
(Phys.org)—Molecules are rarely found alone. In the real world, they are often networked to each other through hydrogen bonding or are bound to other molecules in the surrounding environment. One way to study an individual molecule is to trap it within a fullerene. A fullerene is an all-carbon, spherical molecule with carbons networked like the stitches of a soccer ball. The interior of the fullerene sphere is large enough to house small molecules, such as water or hydrogen gas.
In a recent study, Andrea Krachmalnicoff, et al. from the University of Southampton, the University of Nottingham, and Institutions in France and Estonia have trapped hydrogen fluoride in the cavity of a C60 fullerene (HF@C60). Their characterization studies reveal that HF breaks the fullerene's icosahedral symmetry and that the fullerene cage serves to shield the strongly polar molecule. Their work appears in Nature Chemistry.
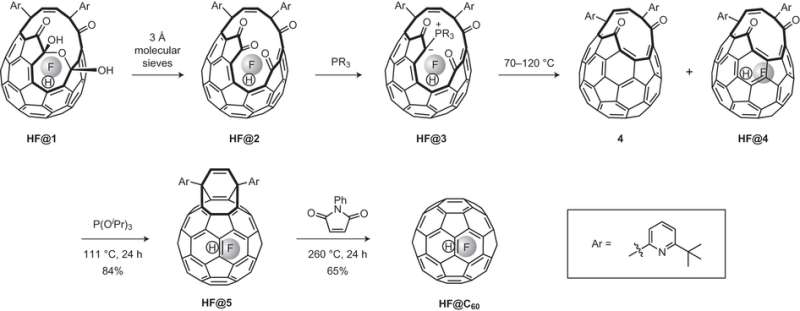
Researchers have been able to trap molecules within the fullerene cavity using a process called "molecular surgery." As the name implies, the fullerene molecules is chemically "cut open" and a small molecule is forced into the spherical cavity. The hole is then chemically "sutured."
Previous research by this group demonstrated the successful encapsulation of HF in an open-cage fullerene; however attempts to suture the open-cage resulted in the HF molecule escaping. The current study reports the successful encapsulation of HF by reacting their open-cage product with PPh(Fu)2 ([2-furanyl] phenylphosphine] in the presence of molecular sieves at room temperature. They obtained the pre-cursor product that can be sutured using methods employed in the synthesis H2O@C60 (i.e., reduction with triisopropylphosphite followed by reaction with N-phenyl-maleimide).
X-ray studies of HF@C60 indicated some distortion of the fullerene cage but because the distortions were not outside of standard deviation values, the results were inconclusive. UV and electrochemical studies, however, did show that the HF molecule does not seem to show an electrostatic interaction with the C60 cage.
NMR studies using 1H, 13C, and 19F NMR as well as solid state 1H and 13C NMR provided more compelling information on the behavior of HF. The J coupling for 1H-19F confirm that there is a single molecule of HF within the C60 cavity.
Krachmalnicoff, et al. then used inelastic neutron scattering and infrared absorption at low temperature to gain insight into the molecule's intrinsic properties. Their findings provide evidence that HF's rotational, vibrational, and translational motion is quantized with all transitions originating from the ground state. These studies showed that in the solid state, HF interact with the C60 molecule both by breaking its icosahedral symmetry and through dipole interactions with the interior of the cage.
Hydrogen fluoride has a large dipole moment. Using capacitance studies to find the dielectric constant at low temperatures, Krachmalnicoff, et al. found that C60 shields the dipole such that the dipole moment for encapsulated HF is about 25% of the calculated dipole moment for HF.
Overall, this research provides some interesting information on the properties of a single-molecule HF. Additional studies are needed to investigate why HF@C60 breaks the endofullerene's icosahedral symmetry in the solid state as well as further investigation into HF@C60's electronic properties.
More information: Andrea Krachmalnicoff et al. The dipolar endofullerene HF@C60, Nature Chemistry (2016). DOI: 10.1038/nchem.2563
Abstract
The cavity inside fullerenes provides a unique environment for the study of isolated atoms and molecules. We report the encapsulation of hydrogen fluoride inside C60 using molecular surgery to give the endohedral fullerene HF@C60. The key synthetic step is the closure of the open fullerene cage with the escape of HF minimized. The encapsulated HF molecule moves freely inside the cage and exhibits quantization of its translational and rotational degrees of freedom, as revealed by inelastic neutron scattering and infrared spectroscopy. The rotational and vibrational constants of the encapsulated HF molecules were found to be redshifted relative to free HF. The NMR spectra display a large 1H–19F J coupling typical of an isolated species. The dipole moment of HF@C60 was estimated from the temperature dependence of the dielectric constant at cryogenic temperatures and showed that the cage shields around 75% of the HF dipole.
Journal information: Nature Chemistry
© 2016 Phys.org