The Ouzo Effect under the magnifying glass
Pour some water into your glass of ouzo or pastis, and the beverage will change from transparent to milky: this is the well-known 'Ouzo effect'. But what will happen if you simply place a drop of ouzo on a surface and wait? Scientists of University of Twente's Physics of Fluids group have studied the phenomena taking place, they distinguish four 'life phases' of the drop, within no more than a quarter of an hour. The results are published in the the Proceedings of the National Academy of Sciences (PNAS) of July 14.
Ouzo is a transparent alcoholic beverage consisting of water, alcohol and anise oil. The solubility of the oil varies with the alcohol-water ratio. Pouring water to the fluid, causes the oil solubility to decrease. The oil starts forming nano-size droplets (nucleation) which will, in turn, form larger micro droplets scattering the light. At that moment, the liquid has its well-known milky appearance.
Rapid movement
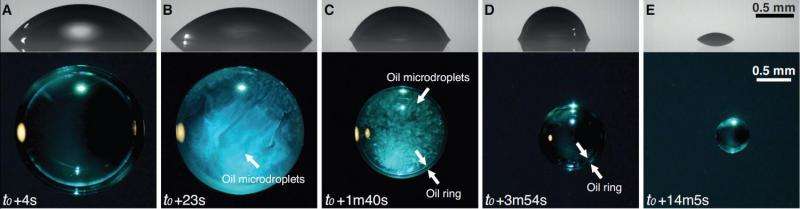
By just placing a drop of ouzo on a surface that is hydrophobic, the phenomena can be studied as well. At first, the drop is transparent. But alcohol, being the most volatile component, starts evaporating first, leaving relatively more water in the drop. The alcohol preferably evaporates at the rim of the drop: it is there that the onset of the ouzo effect takes place. Within the whole drop, a rapid movement will start. This convection is caused by differences in surface tension. The 'Marangoni effect' can also be observed when 'tears' of port wine form at the inside of a glass. Caused by the rapid movement, the ouzo effect that started at the rim, will spread out through the whole of the drop. Until then, the shape of the drop is still spherical, as expected.
Transparent again
This changes in a remarkable way, when the oil starts moving towards the rim and shows an angle between the sphere and the surface: the droplets together form a ring (by coalescence) on the outside of the drop. After a while, all alcohol has evaporated, and the liquid is transparent again. Water, in the meantime, is evaporating as well, causing the ring to grow towards the center of the drop, leaving just a drop of anise-oil in the end. These four phases take place within a quarter of an hour, at room temperature.
The first three phases, involving all of the complex physics inside the drop, don't take long: within two minutes, alcohol evaporates, rapid movement starts as well as a change of the shape caused by the oil ring. The rest of the evaporation, until only a tiny drop of anise oil is left, takes about twelve minutes.
Liquid-liquid extraction
Using the separation mechanisms occurring in a ternary mixture like ouzo, the best conditions can be found for extracting one of the components, for example: liquid-liquid extraction. This can be applied in medical diagnostics, for example. The evaporation process can, furthermore, be controlled by introducing surfaces with varying hydrophobic properties. The research also has an impact on techniques like ink jet printing and 3D printing, using complex fluids.
Apart from that, the results give new insights in the behavior of fluids used in energy technology and catalysts. The Physics of Fluids group of Prof Detlef Lohse takes part in the Dutch national project Multiscale Catalytic Energy Conversion (MCEC).
More information: 'Evaporation-triggered microdroplet nucleation and the four life phases of an evaporating Ouzo drop' by Huanshu Tan, Christian Diddens, Pengyu Lyu, Hans Kuerten, Xuehua Zhang en Detlef Lohse, is published by the Proceedings of the National Academy of Sciences (PNAS), July 14. www.pnas.org/cgi/doi/10.1073/pnas.1602260113
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Twente




















