A new key to understanding molecular evolution in space
Scientists at Hokkaido University have revealed temperature-dependent energy-state conversion of molecular hydrogen on ice surfaces, suggesting the need for a reconsideration of molecular evolution theory.
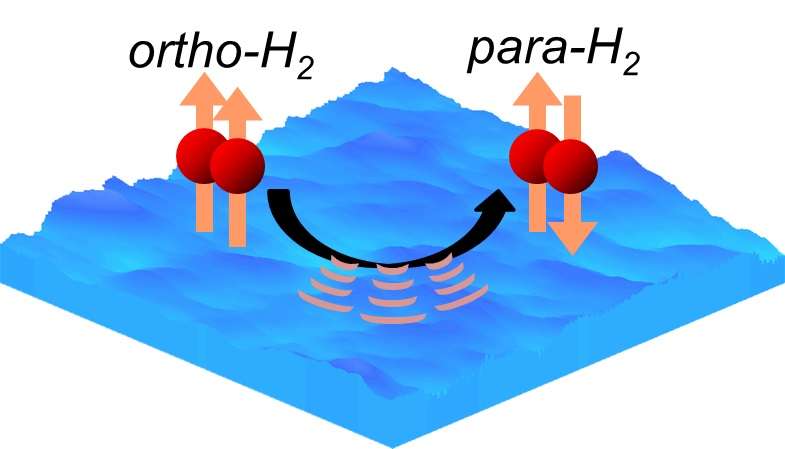
Molecular hydrogen, the most abundant element in space, is created when two hydrogen atoms bond on minute floating ice particles. It has two energy states: ortho and para, depending on the direction of proton spins. Ortho-hydrogen converts to para-hydrogen on extremely low temperature ice particles, though its mechanism remained unclear.
When molecular hydrogen is released from tiny ice particles in space, the particular state of its energy plays a key role in molecular evolution—the process of generating a wide range of molecules over a long period of time in space.
In the study, the researchers developed a special system that could detect the ortho/para ratio of molecular hydrogen on artificial ice particles. The study discovered that the ratio of the ortho-to-para conversion rate (as time passed) was dramatically different in the relatively small temperature range of between -264C and -257C, thus, in a world first, unraveling the conversion mechanism. Ortho-hydrogen converts to para-hydrogen by releasing energy to the ice, in a temperature dependent manner.
Until now, the energy conversion rate was believed to be identical regardless of the temperature of ice particles—a theory that has been scotched by the new research. The finding will likely prompt scientists to rebuild theories of molecular evolution, opening new horizons in studies of molecular formation and molecular evolution.
The research article was selected as an "Editors' Suggestion" in the scientific journal Physical Review Letters.
More information: Hirokazu Ueta et al. Surface Temperature Dependence of Hydrogen Ortho-Para Conversion on Amorphous Solid Water, Physical Review Letters (2016). DOI: 10.1103/PhysRevLett.116.253201
Journal information: Physical Review Letters
Provided by Hokkaido University