Diarrhoea pathogen modifies the surface of intestinal cells, enabling bacteria to colonize it more easily
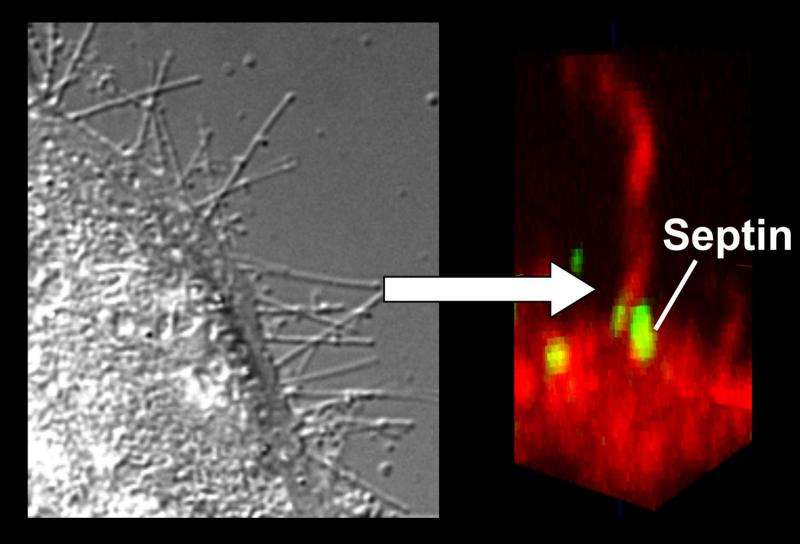
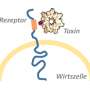
The ingestion of antibiotics often damages the intestine's natural flora. This prevents it from keeping pathogens under control; diarrhoea and intestinal inflammation are the result. Clostridium difficile is one of the pathogens that attack intestinal cells through toxins. One of the things the bacteria does is cause a fine network of protrusions to form on the surface of the intestinal cells, thus enabling further bacteria to settle there. Prof. Dr. Dr. Klaus Aktories and Dr. Carsten Schwan and their research group at the Institute of Experimental and Clinical Pharmacology and Toxicology of the University of Freiburg have demonstrated how the toxin CDT of C. difficile bacteria forms these cellular protrusions. The scientists published their research results in the scientific journal Proceedings of the National Academy of Sciences (PNAS). "By researching the CDT toxin, we are able to better understand how intestinal inflammations are caused by pathogens and how they develop," says Aktories. "We can also use the toxin as a tool for shedding light on fundamental physiological processes."
Especially aggressive bacteria of the C. difficile species produce toxins that destroy the cellular structure of intestinal cells. This inhibits the contacts between intestinal cells as well as their function as barriers, which can cause diarrhoea and inflammation. Two important elements of cellular structure are actin and microtubules, which play a key role in preserving the cell's form, its function as a barrier and its cellular movement processes. The CDT toxin of C. difficile modifies actin, thereby blocking its chain formation and disrupting its normal function. One result of this is that microtubule chains form more easily, which then multiply to such a degree that many cellular protrusions evolve. These form a network on the intestinal cell's surface and promote the contact of bacteria with the host cell.
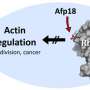
How CDT forms these cellular protrusions was not known until now. The scientists from the University of Freiburg have demonstrated that the influence of the toxin on the cooperation between the two scaffold proteins actin and tubulin depends on a third element: septins. There are up to 13 different septins in a human cell. They interact with each other and can form chains, rings or bands in a process called polymerization. CDT modifies actin in such a way that the septins can no longer bind to the actin and instead migrate to the cellular membrane. Here, they form collar-like septin polymers, in which tube-shaped microtubules grow. Septins interact directly with the tips of growing microtubules and thus function as guides for the growth of these structures.
The research done by the University of Freiburg team also provides insight into the development of septin collar formations. The Cdc42 and Borg proteins regulate the transport of septins to the membrane and are thus a necessary condition for the collar formations to be able to develop. Similar to how the toxin CDT causes protrusions to form, septins also play a role in the human nervous system by forming nerve protrusions called neurites. As in the first case, actin, microtubules and septins also interact closely here to form microscopically similar structures. Researching this toxin therefore helps us to better understand fundamental processes in the human body.
More information: Thilo Nölke et al. Septins guide microtubule protrusions induced by actin-depolymerizing toxins liketransferase (CDT), Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1522717113
Journal information: Proceedings of the National Academy of Sciences
Provided by Albert Ludwigs University of Freiburg