Tiny alpaca-derived antibodies point to targets preventing viral infection
Whitehead Institute scientists have determined how to use alpaca-derived, single-domain antibody fragments (also called VHHs or nanobodies) to perturb cellular processes in mammalian cells, including the infection of human cells by influenza A virus (IAV) and vesicular stomatitis virus (VSV). With improved knowledge of protein activity, scientists can tease apart the roles individual proteins play in cellular pathways, understand how disease corrupts cellular function, and begin to design interventions to rectify such aberrations.
Until now, researchers have relied largely on genetic approaches or small molecules to inhibit protein function. However, these methods' usefulness has limits—genetic alterations may cause unintended phenotypes. Only about 15% of proteins are "druggable" using small molecules.
"Our method is an interesting and, in my opinion, an important addition to the toolbox of the molecular biologist," says Whitehead Member Hidde Ploegh, who is also a Professor of biology at Massachusetts Institute of Technology and an affiliate member of the Koch Institute for Integrative Cancer Research at MIT. "The approach allows you to work in a wild-type protein environment—you don't tinker with the host's protein structure or the genetic makeup of the cell you wish to study, but rather you add a highly specific perturbant."
Ploegh's lab has devised a screening strategy that employs VHHs or nanobodies. These molecules are small, highly specific in what they recognize, and sturdy enough to function in the environment of the cytosol. In earlier work, the Ploegh lab used nanobodies to image the immune system's function in real-time. Working with Whitehead Fellow Sebastian Lourido's lab, VHHs made by the Ploegh lab helped decipher the mode of action of a key enzyme used by the Toxoplasma gondii parasite to invade cells.
In the current line of research, described online in the journal Nature Microbiology, scientists led by postdoctoral researcher Florian Schmidt have developed a rational screening approach that led to the identification of nanobodies that interfere with the ability of IAV and VSV to infect cells.
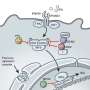
First, the scientists created nanobodies against IAV or VSV by injecting alpacas with inactivated viruses. Millions of DNA sequences, amplified from the immunized alpacas, were inserted into lentiviruses to enable expression of VHHs in the cytosol of human cells. The transduced human cells were then challenged with IAV or VSV. Any surviving cells must have produced a VHH that interferes with virus replication. Indeed, of the millions of cells transduced, about 260 contained nanobodies that protected the cells against either virus and reduced viral infection by more than 80%. When Schmidt analyzed these hits, he found that the nanobodies jammed the viruses' infection machinery using tactics specific to each virus—anti-IAV VHHs targeted the viral nucleoprotein NP, while the anti-VSVs recognized the viral nucleocaspid N.
Using a similar, nanobody-based method, Schmidt determined the role of the adaptor protein ASC in inflammasome assembly in myeloid cells, but he envisions even broader applications for such screens.
"This technique is a very rapid way of identifying inhibitors of essentially any biological process," he says. "And it allows us to look at all the surfaces of a collection of proteins that we're interested in and find the sites that are important for protein function."
By stabilizing their target molecules, nanobodies act as crystallization chaperones, which allow scientists to more easily solve the proteins' structure. The sites where VHHs bind to proteins are also potential drug targets, as these locations impair the proteins' activity.
More information: Phenotypic lentivirus screens to identify functional single domain antibodies, Nature Microbiology, DOI: 10.1038/nmicrobiol.2016.80
Journal information: Nature Microbiology
Provided by Whitehead Institute for Biomedical Research