October 26, 2015 feature
Tiny sponges behave in a counterintuitive way when adsorbing water
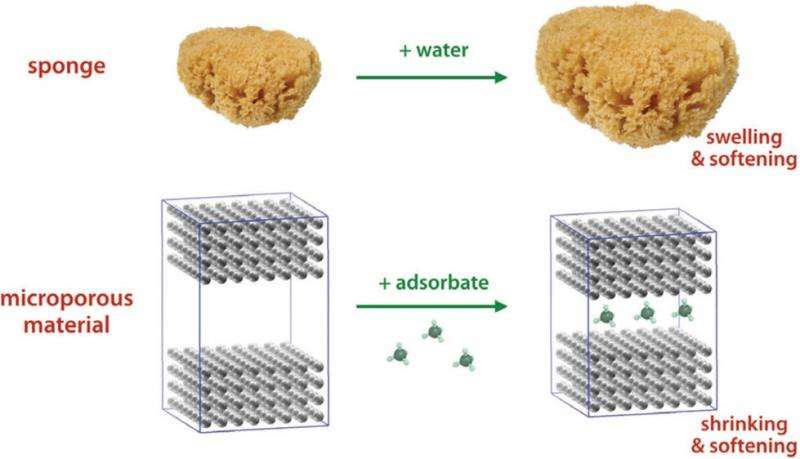
(Phys.org)—When a kitchen sponge adsorbs water into its pores, it softens and expands. Now in a new study published in The Journal of Physical Chemistry Letters, scientists have discovered that, when microporous materials adsorb fluid, they initially soften but then stiffen as they adsorb more fluid. It's as if soaking your kitchen sponge in water eventually caused it to harden.
"When materials have pore spaces that are really small (close to molecular dimensions), the physics is totally different than what we know from an everyday perspective," coauthor François-Xavier Coudert, a researcher at CNRS & Chimie ParisTech, told Phys.org. "We need to think differently at the nanometer scale, and not make assumptions based on our intuition."
The counterintuitive softening-then-stiffening behavior in microporous materials corresponds to a contraction-then-expansion behavior. While macroporous materials always expand when adsorbing a fluid, it's been known since the 1940s that microporous materials initially shrink when adsorbing a small amount of fluid, and then start to expand when adsorbing a larger amount of fluid. The initial contraction phase can be explained by microscale attractive forces that pull the molecules in the microporous material and the fluid together, causing the material to shrink.
It might be expected that microporous materials would stiffen when shrinking and soften when expanding, since this is what generally happens in macroporous materials like sponges. But the researchers observed the opposite: microporous materials soften as they shrink, and then stiffen as they expand.
The scientists demonstrated the surprising new behavior in simulations of a real material and two pore shapes (slits and lozenge-shaped), which suggests that this response might be general to all pore shapes. However, experimental studies are needed to test this possibility and analyze in more detail what controls this behavior. Most likely, this means looking not at just the pores but at the entire material.
"People tend to think of materials as a 'matrix' inside of which molecules can get and within which they move around," Coudert said. "But the material itself is strongly affected by the presence of those molecules within its pores. You have to consider the system as a whole: porous-material-plus-guest phase."
Although surprising, the new findings could help explain some recent counterintuitive experimental results. In one recent experiment, for example, a microporous material initially softened after adsorbing fluid, just as the new simulations predict. In another recent experiment, the scientists found that the stability of a microporous material decreased after adsorbing water. If the decrease in stability is related to softening during the initial contraction phase, then it would mean that adsorption can have a significant impact on the stability of microporous materials. Understanding stability is crucial for practical applications at the industrial level.
Overall, research on fluid adsorption in microporous materials could have applications in a variety of areas, including controlled drug delivery, biosensing, ion exchange, and using catalysts to accelerate chemical reactions. Areas such as these could one day benefit from a better understanding of the surprising new behavior, which the researchers hope to further explore in the future.
"On the experimental side, we will be looking for more direct evidence of this behavior that we are underlining," Coudert said. "We will also continue our theoretical work and see how that softening-stiffening behavior occurs in framework materials whose structure is more complicated at the microscopic scale. For example, in very anisotropic materials [whose properties depend on direction], do all directions behave similarly or not under adsorption? What dictates this?"
More information: Félix Mouhat, et al. "Softening upon Adsorption in Microporous Materials: A Counterintuitive Mechanical Response." The Journal of Physical Chemistry Letters. DOI: 10.1021/acs.jpclett.5b01965
Journal information: Journal of Physical Chemistry Letters
© 2015 Phys.org










.jpg)


.jpg)