Alert to biologists: Ribosomes can translate the 'untranslated region' of messenger RNA
In what appears to be an unexpected challenge to a long-accepted fact of biology, Johns Hopkins researchers say they have found that ribosomes—the molecular machines in all cells that build proteins—can sometimes do so even within the so-called untranslated regions of the ribbons of genetic material known as messenger RNA (mRNA).
"This is an exciting find that generates a whole new set of questions for researchers," says Rachel Green, Ph.D., a Howard Hughes Medical Institute investigator and professor of molecular biology and genetics at the Johns Hopkins University School of Medicine. Chief among them, she adds, is whether the proteins made in this unusual way have useful or damaging functions and under what conditions, questions that have the potential to further our understanding of cancer cell growth and how cells respond to stress.
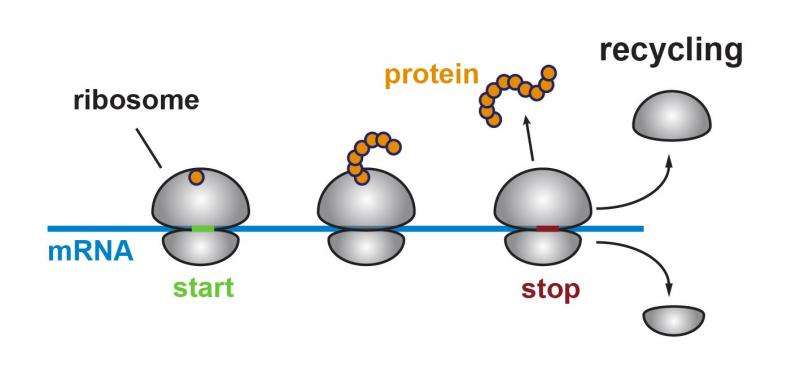
In a summary of the findings in yeast cells, to be published Aug. 13 in the journal Cell, Green and her team report that the atypical protein-making happens when ribosomes fail to get "recycled" when they reach the "stop" signal in the mRNA. For reasons not yet understood, Green says, "rogue" ribosomes restart without a "start" signal and make small proteins whose functions are unknown.
Ribosomes are made out of specialized RNA molecules (DNA's chemical cousin) that work together with proteins to read instruction-bearing mRNAs and "translate" their message to create proteins. Each mRNA begins with a "start" code, followed by the blueprint for a specific protein, followed by a "stop" code. And then there's a segment of code that has always been called the "untranslated region," because scientists never saw it translated into protein.
But no longer, according to Green and postdoctoral fellow Nicholas Guydosh, Ph.D., who, along with a team at the National Institute of Child Health and Human Development, began the project out of curiosity about a yeast protein called Rli1.
Previous studies had shown that Rli1 can split ribosomes into their two component parts once they encounter a stop code and are no longer needed. This "recycling" process, they say, disengages a ribosome from its current mRNA molecule so that it's available to translate another one. But it was unclear whether Rli1 behaved the same way in live cells.
To find out, the researchers deprived living yeast cells of Rli1, predicting that translation would slow down as ribosomes piled up at stop codes. To "see" where the ribosomes were, the team added an enzyme to the cells that would chew up any exposed RNA. The RNA bound by ribosomes would be protected and could then be isolated and identified. As predicted, the depletion of Rli1 increased the number of ribosomes sitting on stop codes. But they also saw evidence of ribosomes sitting in the untranslated region, which they called a surprise.
To find out if the ribosomes were actually reading from the untranslated region to create proteins, the team inserted genetic code in that region for a protein whose quantity they could easily measure. Cells with Rli1 didn't make the protein, but cells missing Rli1 did, proving that their ribosomes were indeed active in the untranslated region.
Further experiments showed that the ribosomes weren't just continuing translation past the stop code to create an extra-long protein. They first released the regularly coded protein as usual and then began translation again nearby.
"It seems like the ribosomes get tired of waiting to be disassembled and decide to get back to work," says Guydosh. "The protein-making work that appears right in front of them is in the untranslated region."
As noted, the purpose of these many small proteins is unknown, but Green says one possibility stems from the fact that ribosomes increase in the untranslated region when yeast are stressed by a lack of food. "It's possible that these small proteins actually help the yeast respond to starvation, but that's just a guess," she says.
Because ribosomes are essential to create new proteins and cell growth, Green notes, scientists believe the rate at which cells replicate is determined, at least in part, by how many ribosomes they have. Cells lacking Rli1 can't grow because their ribosomes are all occupied at stop codes and in untranslated regions. Thus cancer cells increase their levels of Rli1 in order to grow rapidly.
"We didn't understand previously how important ribosome recycling is for the proper translation of mRNA," says Green. "Without it, ribosomes are distracted from their usual work, which is crucial for normal cell maintenance and growth. This finding opens up questions we didn't even know to ask before."
Journal information: Cell
Provided by Johns Hopkins University School of Medicine