Oxygen atoms create detailed architectures in uranium dioxide, altering our understanding of corrosion
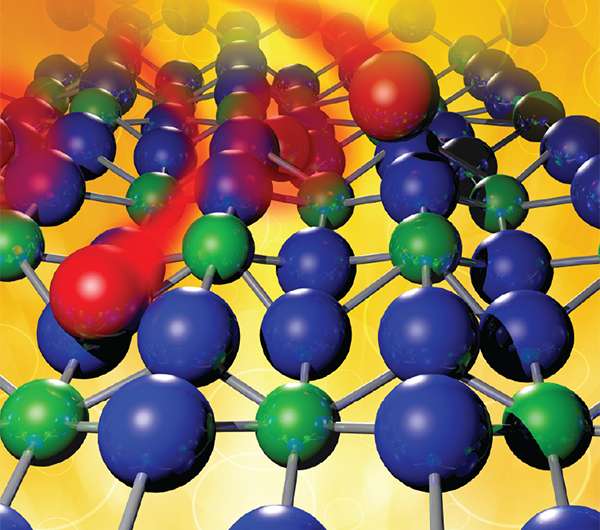
Corrosion follows a different path when it comes to uranium dioxide, the primary component of the rods that power nuclear reactors, according to a new study by scientists at the Pacific Northwest National Laboratory, University of Chicago, and the Stanford Synchrotron Radiation Lightsource. In uranium dioxide, the oxygen atoms-key corrosion creators-do not diffuse randomly through the material. Rather, the oxygen atoms settle into the third, sixth, ninth, etc., layers. They space themselves within the layers and alter the structure by causing the layers of uranium atoms above and below to draw closer to the oxygen. The oxygen atoms essentially self-assemble into a highly structured array.
Oxygen's interactions can extensively corrode materials, whether it is a car in a field or a fuel canister in a nuclear reactor. Under certain conditions, oxygen corrodes fuel rods and causes them to swell by more than 30 percent, creating problems during both routine operations and emergency situations. Also, this swelling can be a problem for long-term storage of nuclear waste. The study shows atomic-level changes counter to those shown by the classical diffusion model that states most of the oxygen atoms are near the surface. The new study gives scientists accurate information to understand the start of corrosion, possibly leading to new ways to avoid corrosion-related failures.
When uranium dioxide is exposed to oxygen, the classical diffusion model shows the oxygen randomly moving into the uranium. Specifically, the oxygen atoms settle on the surface and grab two electrons from one uranium atom or one electron from each of two on the way in.
That's not the case.
Instead, oxygen atoms pull in a bit of the negative charge from the nearest uranium atoms, from the next nearest, and from the next-next nearest. This shell of slightly oxidized (more positively charged) uranium forms a protective sphere around the oxygen. The next oxygen atom in moves just far enough away to get electrons from untouched atoms. As more oxygen enters, the atoms form an organized structure.
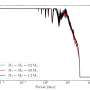
The team from PNNL, University of Chicago, and SSRL made their discovery by combining X-ray scattering and spectroscopy experiments to determine the positions of the uranium atoms. The results showed the uranium atoms contracting every third layer. As no model explained why and the X-ray scattering was unable to "see" the oxygens, the team calculated the positions of the atoms using two supercomputers. They found that the oxygen atoms went into every third layer, spaced themselves out within the layer (occupying about a quarter of the layer) and caused the nearby uranium layers to move.
Why the third layer? This layer is most thermodynamically favorable.
Why is the arrangement of quarter occupancy every three layers the most thermodynamically favorable? Because of the uranium atoms' ability to donate a small fraction of electronic charge to the corroding oxygen atoms, creating the oxidized sphere of three shells of uranium atoms around each oxygen.
"Nobody had ever seen something like this before - where the oxygen comes in and self organizes every three layers," said Dr. Anne Chaka, a geochemist at PNNL who conducted the quantum mechanical modeling. "It took quantum mechanical modeling on large supercomputers to understand what the electrons were doing and how that drove the spacing of the oxygen atoms."
More information: "UO2 Oxidative Corrosion by Nonclassical Diffusion." Physical Review Letters 114:246103. DOI: 10.1103/PhysRevLett.114.246103
Journal information: Physical Review Letters
Provided by Pacific Northwest National Laboratory