Researchers convert ethane to ethanol with an efficiency that could cut natural-gas refining costs
The Molecular Foundry is a national user facility, one of the U.S. Department of Energy's (DOE's) nanoscale science research centers. It is located at Lawrence Berkeley National Laboratory and supported by the DOE's Office of Basic Energy Sciences. The Molecular Foundry serves hundreds of academic, industrial and government scientists from around the world each year who come to the Foundry to perform multidisciplinary research beyond the scope of their own laboratories and to develop science and technology strategies in energy, electronics, materials science, and biology. The world-class scientists at the Molecular Foundry have expertise across the state-of-the-art, unique instrumentation in a broad range of disciplines.
Refining natural gas into an easy-to-transport, easy-to-store liquid so far has been a challenge. But now, a new material, designed and patented by researchers working at the Molecular Foundry nanoscience research center, is making this process a little easier. Their findings could pave the way for the adoption of cheaper, cleaner-burning fuels.
Natural gasses, like ethane and methane, are hard to store and transport. However, by converting them to liquids specifically, liquid alcohols, they could be more easily stored, transported and used as fuels. Ethanol, the liquid form of ethane, is a particularly attractive form of liquid fuel because it burns cleaner and provides greater energy than its alternatives. Unfortunately, current methods for making ethanol from ethane require extreme heat (approximately 400-600 °F), making it a very expensive fuel.
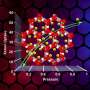
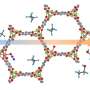
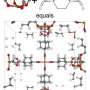
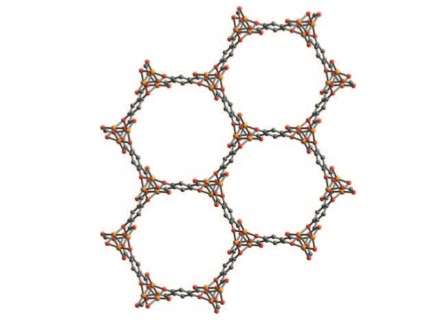
To lower the temperature, and thus lower costs, a research team at the Molecular Foundry created a collection of tiny cages, a metal-organic framework (MOF) which speeds the chemical reaction that turns ethane into ethanol. In addition to their catalyzing ability of quickly turning one chemical into another, MOFs can also act as a chemical filter, capturing and holding selected molecules. By using a specially designed MOF—one in which a kind of iron was added inside the tiny molecular cages—the researchers were able to reduce the need for extreme heat, converting ethane to alcohol at just 167°F. This reduces costs involved in making the preferred form of fuel.
Provided by US Department of Energy