A new secondary explosive with high thermal stability
Ludwig Maximilian University of Munich chemists have developed a new secondary explosive which has a significantly higher thermal stability than the commonly used pentaerythritol tetranitrate (PETN) and is therefore easier and safer to handle.
Explosive chemicals are designed to store large amounts of energy and release it instantaneously on demand. Yet they must also be sufficiently stable, both to adventitious detonation and to decomposition, to permit long-term storage with minimal risk to the security of personnel. PETN (pentaerythritol tetranitrate), a so-called nitrate ester, is a widely utilized explosive compound, but its sensitivity to friction and impact leaves a lot to be desired. A research group led by Professor Thomas M. Klapötke in the Department of Chemistry at LMU Munich has now developed a new derivative of PETN that is thermally more stable than its parent. Synthesis and characterization of the new compound is described in the latest issue of the European Journal of Organic Chemistry.
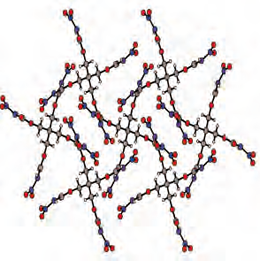
Unlike PETN, the new material does not belong to the nitrate esters, and was synthesized by nitration of a so-called carbamate in a two-step scheme. The reaction product, pentaerythritol tetranitrocarbamate (PETNC for short), is thus classified as a nitrocarbamate. The primary carbamate itself was synthesized from the corresponding alcohol under mild conditions, using chlorosulfonylisocyanate (CSI) as a catalyst. "This method has a number of advantages – the reaction is very rapid, for one thing – and it could provide a synthetic route to a whole range of new materials," says Professor Klapötke.
Like PETN, PETNC is a secondary explosive, and requires the use of a primary explosive as a detonator. Indeed, the laboratory tests carried out by the LMU researchers revealed that PETNC has a higher thermal stability than PETN. While the latter melts at 141°C and begins to decompose at 165°C, PETNC remains stable up to a temperature of 196°C.
"PETNC is not only less susceptible to heat, it is also significantly less sensitive to impact than PETN, and not at all sensitive to friction. So it should be much easier and safer to handle in practice," Klapötke adds. In order to test its performance as an explosive, the new compound was packed into a steel ring bolted to an aluminum block, and a commercially available detonator was used to set it off. By measuring the degree of deformation of the underlying aluminum block, the researchers determined that PETNC's detonation parameters are only slightly less favorable than those of PETN, in good agreement with theoretical calculations based on its chemical structure.
More information: Axthammer, Q. J., Krumm, B. and Klapötke, T. M. (2014), "Pentaerythritol-Based Energetic Materials Related to PETN." Eur. J. Org. Chem.. doi: 10.1002/ejoc.201403265
Journal information: European Journal of Organic Chemistry
Provided by Ludwig Maximilian University of Munich