Scientists create mouse model to accelerate research on Ebola vaccines, treatments
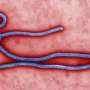
In the war against Ebola, one important hurdle has just been cleared – by a mouse.
A study published in the latest issue of Science details how researchers from UNC and their colleagues at NIH Rocky Mountain National Laboratory and the University of Washington have developed a new genetic strain of mice that will significantly improve opportunities to test the initial efficacy of potential vaccines and treatments for Ebola and other emerging pathogens.
The study, titled "Host genetic diversity enables Ebola hemorrhagic fever pathogenesis and resistance," describes how these researchers solved an important challenge facing any scientific team seeking remedies to Ebola.
Mouse lines traditionally used for pharmacological and medical research do not develop human-like Ebola disease, including the severe symptoms that can prove fatal in humans. The study's co-authors were able to breed together genetic mouse variants and successfully test a strain of mice to permit active research on potential Ebola vaccines and treatments in model systems that more accurately reflect the human condition.
William Fischer II, MD, an assistant professor of medicine who has treated Ebola patients in Africa, said, "A basic understanding of how our genetics influence susceptibility to viral infections and affect disease development is absolutely critical to creating much needed therapeutic interventions." Fischer, who was not part of this research project, added, "We can decrease Ebola fatality rates with intensive critical care but this is difficult to do in places with limited human and material resources. Rationally designed treatments and vaccines are desperately needed."
Science paper co-author, Martin Ferris, PhD, a research assistant professor of genetics in the UNC School of Medicine, said, "Public perception of Ebola infection typically focuses on the high mortality rate following hemorrhagic fever, but Ebola actually produces a range of disease symptoms. During an outbreak, it is often difficult to assess the role that genetic variation plays in determining disease severity in people. And if we're going to develop treatments, then we need to know about this genetic variation."
Ferris and colleagues bred and tested different mouse strains from the "Collaborative Cross" for susceptibility to Ebola. The Collaborative Cross, which is housed at and distributed by UNC, is a population of genetically diverse mice that was developed by five institutions, including UNC, so that researchers can assess the role of genetic variation in a wide spectrum of medically relevant phenotypes – observable physical and biochemical characteristics and traits of an organism.
"Since the Collaborative Cross was designed to better model human genetic diversity, it has proven to be a powerful system for studying how genetic variation affects susceptibility to a number of emerging pathogens, including Ebola virus," said study co-author Mark Heise, PhD, professor of genetics at the UNC School of Medicine.
These studies, which were conducted at the NIH Rocky Mountain National Laboratory, demonstrated that the genetic variation of the mice directly led to different disease responses after infection. Importantly, these research groups were able to identify variation within a specific gene that controlled much of the variation in disease severity between individual mice. Because this gene also is variable within the human population, the results of this research point to a potential target for therapeutics in combating Ebola in people.
"In essence, we were able to produce a novel platform for rapidly developing new mouse models that replicate human disease outcomes when infected with Ebola as well as other important emerging human pathogens in the future," said study co-author Ralph Baric, PhD, professor of epidemiology at the UNC Gillings School of Global Public Health. "This could accelerate and improve studies on potential vaccines or treatments while lowering overall costs."
This work is part of a larger collaboration between the UNC Department of Genetics, the UNC Gillings School of Global Public Health, University of Washington Department of Microbiology, NIH Rocky Mountain National Laboratories, and the Oregon Health Sciences University to understand how genetic variation affects susceptibility to a range of viral diseases.
More information: "Host genetic diversity enables Ebola hemorrhagic fever pathogenesis and resistance." Science DOI: 10.1126/science.1259595 Published Online October 30 2014
Journal information: Science