Method of nickel-carbon heterofullerenes synthesis presented
Scientists from several British, Spanish and Russian research centers (MIPT, Institute for Spectroscopy RAS, Kurchatov Institute and Kintech Lab Ltd) have come up with a method of synthesizing a new type of nickel-carbon compound. The article titled "Formation of nickel-carbon heterofullerenes under electron irradiation" has been published by Dalton Transactions and is available as a pre-print at Arxiv.org. The first author of the article is Alexander Sinitsa, an MIPT student, and the leading author is Andrey Popov (Institute for Spectroscopy RAS, 1989 MIPT graduate).
Heterofullerenes are hollow molecules with a nearly-spherical shape, which, unlike the typical fullerenes, contain atoms of elements other than carbon. Such compounds were synthesized quite a while ago, in 1991, but till now no heterofullerenes containing nickel, or any other transition metal, have been obtained. Yet, as the authors point out in their article, transition metals are now being studied as catalysts in the synthesis of carbon nanotubes and graphene.
"I'd like to emphasize that the majority of calculations have been performed by a student. Hopefully, students regularly visit the MIPT site and get inspired by their colleagues' successes. If you are especially interested in the role of MIPT graduates in research, then I can tell you that Irina Lebedeva graduated from the Institute in 2008, and Andrey Knizhnik, perhaps in 1999, but I'm not exactly sure about the year. I'd also like to point out that Elena Bichoutskaia (a Saint Petersburg State University Faculty of Physics graduate) is a member of the Russian diaspora abroad, which is typical of international cooperation of Russian scientists," Andrey Popov told the MIPT Press Service.
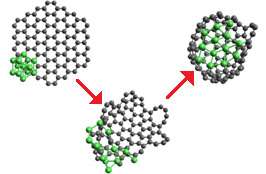
The synthesis of nickel heterofullerenes is supposed to be carried out under electron irradiation, which is used in high-resolution transmission electron microscopy (HRTEM) in order to obtain detailed snapshots showing, if needed, separate atoms. A number of previous experiments conducted by various research groups demonstrated that electronic irradiation can also be applied to synthesize a variety of nanostructures, e.g., one-layer carbon fullerene-filled nanotubes were transformed into two-layer ones.
Using the latest data obtained from the HRTEM images and the results of computer modelling by methods of molecular dynamics, the scientists have shown the potential possibility to transform graphene flakes with nickel cluster into nickel-carbon heterofullerene.
The scientists, though, are not sure about the practical application of such heterofullerenes. According to Andrey Popov, "these new-type molecules can reveal some interesting electronic, magnetic, and optic features, or it may be possible to combine them with some organic functional complexes of interest to biologists and physicians. They can also be used to create 3D organic-metallic structures to store hydrogen".
In their work, the researchers developed and applied an authentic algorithm for modelling electron-nanostructure interactions. This allows taking into account both fast (just tens of picoseconds) and slow (lasting for full seconds) processes. The fast processes are associated with electron collisions, and the slow ones relate to molecular relaxation.
Journal information: Dalton Transactions
Provided by Moscow Institute of Physics and Technology