Bacterial nanometric amorphous Fe-based oxide as lithium-ion battery anode material

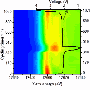
Leptothrix ochracea is a species of iron-oxidizing bacteria that exists in natural hydrospheres where groundwater outwells worldwide. Intriguingly, the bacterium produces Fe3+-based amorphous oxide particles (ca 3 nm diameter; Fe3+:Si4+:P5+~73:22:5) that readily assemble into microtubular sheaths encompassing the bacterial cell (ca 1 μm diameter, ca 2 mm length, Fig. 1). The mass of such sheaths (named L-BIOX : Biogenous Iron Oxide produced byLeptothrix) has been usually regarded as useless waste, but Jun Takada and colleagues at Okayama University discovered unexpected industrial functions of L-BIOX such as a great potential as an anode material in lithium-ion battery.
Since use of the battery that is a powerful electric source for portable electric devices has expanded to a variety of new areas such as transportation and electric power storage, improvement of battery capability and effort to develop new electrode materials have been demanded. The general processes of nanosizing and appropriate surface modification which are required for tuning the battery property are complicated and cost-ineffective. By contrast, L-BIOX is a cost-effective and easily-handled electrode material, since its basic texture is composed of nanometric particles.
The charge-discharge properties of simple L-BIOX/Li-metal cells were examined at current rates of 33.3mA/g (0.05C) and 666mA/g (1C) for voltages of 0 to 3V over 50 cycles (Fig. 2). In addition, electronic and structural changes were microscopically analyzed by TEM/STEM/EELS and 57Fe Mӧssbauer spectroscopy.
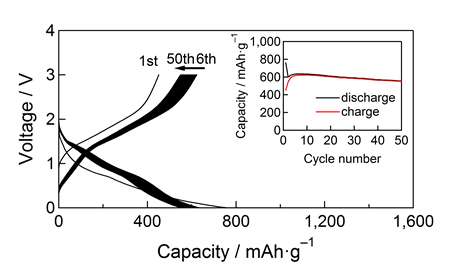
Results showed that L-BIOX exhibited a high potential as an Fe3+/Fe0conversion anode material. Its capacity was significantly higher than the conventional carbon materials. Notably, the presence of minor components of Si and P in the original L-BIOX nanometric particles resulted in specific and well-defined electrode architecture. Since Fe-based electrochemical center is embedded in Si/P-based amorphous texture, an undesirable coagulation of Fe-based center is prevented.
Takada and colleagues proposed a unique approach to develop new electrode materials for Li-ion battery. This is an example showing that the iron oxides of bacterial origin are an unexplored frontier in solid-state chemistry and materials science.
More information: "Bacterial Nanometric Amorphous Fe-Based Oxide: A Potential Lithium-Ion Battery Anode Material." Hideki Hashimoto, Genki Kobayashi, Ryo Sakuma, Tatsuo Fujii, Naoaki Hayashi, Tomoko Suzuki, Ryoji Kanno, Mikio Takano, and Jun Takada. ACS Applied Materials & Interfaces 2014 6 (8), 5374-5378. DOI: 10.1021/am500905y
Journal information: ACS Applied Materials and Interfaces
Provided by Okayama University