Clarification of dynamical process of aluminum surface oxidation
Researchers from the National Institute for Materials Science have presented decisive evidence clarifying the dynamical process of aluminum surface oxidation by using an aligned O2 beam, which was originally developed by the researchers, and thereby settled a dispute which had continued for 20 years regarding the reaction mechanism.
Aluminum is widely used as a corrosion-resistant lightweight material despite its high reactivity for O2 because the dense oxide film that forms on the surface prevents corrosion by oxygen, etc. in the air. In the field of fundamental surface science, O2 adsorption on aluminum surfaces had been investigated for many years as the most representative system of surface oxidation. However, previous experimental and/or theoretical studies on the atomic-scale process of O2 adsorption/dissociation contradict with each other. As a result, the mechanism of this simple surface reaction still remained unclear, in spite of the research extending over more than 20 years.
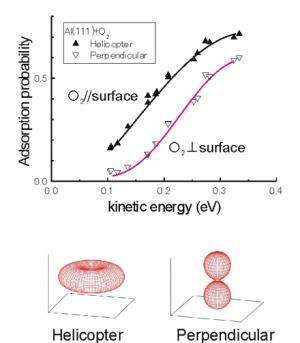
Using an aligned O2 beam developed by the researchers, the team headed by Dr. Kurahashi clarified that the probability of O2 adsorption on an aluminum surface depends strongly on the alignment of the O2 molecular axis. The NIMS researchers demonstrated that low velocity O2 molecules with kinetic energies of 0.1eV or less adsorb only when their axes are nearly parallel to the surface, whereas, O2 molecules in any molecular orientations can adsorb when the kinetic energy exceeds 0.2eV. Until now, O2 molecules with its axis perpendicular to the surface had been considered to adsorb under low energy conditions, and this had long confused the discussion on the reaction mechanism. However, the present research has concluded that this reaction mechanism is not true.
This research also explains the previous experimental results, which had appeared contradictory, and thus elucidated the whole atomic-scale dynamical process of O2 adsorption on an aluminum surface, which had been unclear for many years. Moreover, this research indicates that the slight activation energy difference of 0.1 eV among different molecular orientations needs to be considered for the future study of O2 adsorption on surfaces. O2 adsorption is important not only in the oxidation of the material itself, but also in the catalytic processes happening on the surfaces of fuel cell electrodes, etc. Expensive rare metals such as platinum are used as catalysts that efficiently dissociate O2 molecules. The aligned O2 beam used in this research would be useful not only in reaction analysis, but also in research on substitute catalysts.
These results were published online on June 13 in Physical Review Letters.
Journal information: Physical Review Letters
Provided by National Institute for Materials Science










.jpg)