An unexpected pairing of frustrated molecules
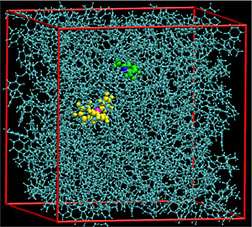
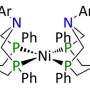
While their shapes frustrate traditional bonding, two unreactive molecules come together and surround themselves within a solvent cage to create a reactive environment and split hydrogen.
Researchers at DOE's Pacific Northwest National Laboratory are revealing the role of the solvent in this process. Splitting a hydrogen molecule into a proton and a hydride ion (H-), known as activating the hydrogen, is vital for sustainable energy production and storage. The pair of molecules is called a frustrated Lewis pair.
"Conventional wisdom says that frustrated Lewis pairs should not be able to activate hydrogen—but they do. We wanted to know why," said Dr. Greg Schenter, a theoretical chemist on this project.
Turning plant material or other renewable resources into fuels requires adding hydrogen without taking up excessive energy. This requirement demands an effective catalyst. These studies provide fundamental insights into the processes that could one day be used to create that catalyst.
Provided by US Department of Energy