Scientists use CLS to identify key protein in stopping viruses
(Phys.org)—Using the Canadian Light Source (CLS) synchrotron, researchers have determined the structure of a key protein that stops viruses from spreading, an important step towards developing new ways of fighting viral diseases.
Viruses like influenza, SARS, Hepatitis C, West Nile fever and polio are small, infectious agents that can spread quickly throughout the body by replicating their genetic code, or ribonucleic acid (RNA).
During the replication process, small proteins are needed to synthesize the virus throughout the body. However some proteins actually inhibit the RNA from replicating, keeping the virus from spreading.
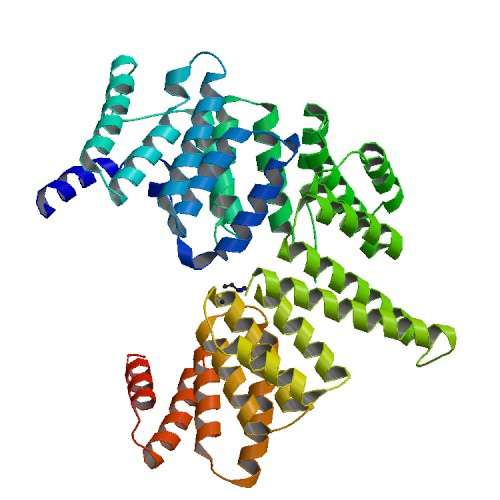
While scientists were aware of the antiviral protein molecules, such as the IFIT protein, they were not certain what their molecular structures looked like and how they reacted with the viral RNA.
Making use of data collected at the CLS, researchers from McGill University and the Austrian Research Center for Molecular Medicine (CeMM) discovered the molecular blueprint behind the IFIT protein. The discovery will help scientists develop new drugs for combatting a wide-range of immune system disorders.
The discovery was made by teams led by Bhushan Nagar, professor of biochemistry at McGill's Faculty of Medicine and Dr. Giulio Superti-Furga at CeMM. The results were published recently in the journal Nature where the article describes how the IFIT protein binds to foreign RNA, allowing the immune system to distinguish "self from non self".
"This discovery of the IFIT protein structure is very rewarding to us at the Canadian Light Source," said Shaun Labiuk, research associate with the Canadian Macromolecular Crystallography Facility (CMCF) at the CLS. "We are always excited when the work we put into helping researchers, and maintaining the smooth operation of our beamlines, makes high-calibre of research like this possible."
The CMCF scientists operate two X-ray crystallography beamlines to conduct high-resolution structural studies of proteins, nucleic acids and other macromolecules.
Since 2006, Researchers have used data collected at the CLS synchrotron to solve crystal structures of 328 new proteins, many of which help researchers develop new ways of combatting diseases.
Journal information: Nature
Provided by Canadian Light Source