Study details dimmer switch for regulating cell's read of DNA code
(Phys.org)—Epigenetics - the science of how gene activity can be altered without changes in the genetic code - plays a critical role in every aspect of life, from the differentiation of stem cells to the regulation of metabolism and growth of cancer cells.
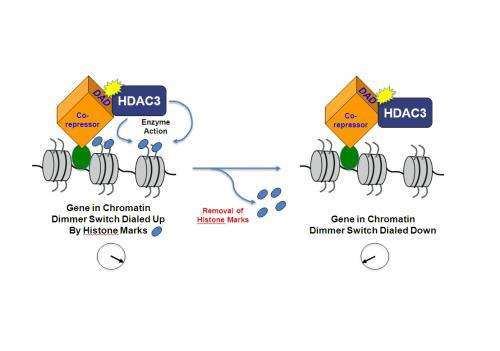
Epigenetic factors act by reworking the structure in which genes reside, called chromatin. Inside chromatin, DNA is wound around proteins called histones. Several new cancer treatments interfere with the function of enzymes that chemically mark the histones to alter the readout of the DNA code and ramp the expression of genes up or down, as if with a dimmer switch. Enzymes called histone deacetylases (HDACs) erase the mark and shut off gene expression.
A team led by Mitchell A. Lazar, M.D., Ph.D., director of the Institute for Diabetes, Obesity, and Metabolism at the Perelman School of Medicine, University of Pennsylvania, has been studying HDAC3 for several years. They discovered that the enzyme activity of HDAC3 requires interaction with a specific region on another protein, which they dubbed the Deacetylase Activating Domain or "DAD." This "nuts and bolts" discovery on the epigenetic control of a person's genome has implications for cancer and neurological treatments.
This domain is found only in proteins that are nuclear receptor corepressors (NCoR1 and NCOR2), which assist receptor proteins in the nucleus to downregulate gene expression.
The team showed that HDAC3 enzyme activity is undetectable in mice bearing mutations in the DAD of both NCOR1 and NCOR2, also called SMRT, despite having normal levels of HDAC3 protein. The findings were published this week in Nature Structural & Molecular Biology.
HDAC3 is required for normal mouse development and tissue-specific functions. In cell culture studies, the HDAC3 protein itself has minimal enzyme activity but gains its histone-deacetylation function from stable association with the DAD.
"We developed a unique mouse model to directly test whether HDAC3 absolutely requires NCOR1 and/or SMRT to be activated," says Lazar. "The answer is yes." The results clearly show that, although tissue levels of HDAC3 are normal in this mouse model, the protein does not have detectable enzyme activity in embryos and various tissues of the engineered mice.
Surprisingly, the engineered mice are born and live to adulthood, whereas genetic absence of HDAC3 is lethal to the mice before they are born. This suggests that HDAC3 may have a deacetylase-independent function which, Lazar says, "is potentially of major importance, because HDAC inhibitors are currently used clinically to treat cancer, and are in clinical development for neurological illnesses and other disorders. We are working hard in the lab to sort this out."
Journal information: Nature Structural & Molecular Biology
Provided by University of Pennsylvania School of Medicine