August 21, 2012 feature
Crystals take a chill pill: A thermomechanical theory of low-temperature melting
(Phys.org) -- Virtual melting is a phase transition phenomenon associated with solid-solid phase transformation and relaxation of nonhydrostatic stresses and other effects in HMX explosives, as well as with crystal-crystal and crystal-amorphous phase transformation under high pressure. Since its initial formulation by Iowa State University Professor Valery I. Levitas and coworkers, virtual melting has been estimated to occur at 100 - 1000 °K below the material’s equilibrium melt temperature. Recently, however, Levitas and University of Texas Associate Professor Ramon Ravelo proposed a new deformation mechanism on which melting can occur at temperatures 4000 °K lower than the equilibrium melt temperature in materials subjected to high deviatoric stresses (where stress components vary by direction, and which control the degree of body distortion) in a shock wave. In addition, they’ve developed a novel thermomechanical theory of melting that predicts extremely large reduction in melting temperature.
Levitas and Ravelo began their investigation when Levitas gave a talk on virtual melting in 2005 at Los Alamos National Laboratory, and there met Ravelo. “Ramon was very critical and asked a lot of questions,” Levitas recounts to Phys.org. “He noted that in atomistic simulations of shock wave propagation in defect-free crystals, it had been observed that disordering occurred at the shock front at temperatures much below the melt temperature at the corresponding shock pressure along some directions – but along others, melting occurred at or above the melting temperature.”
Moreover, Levitas adds, it was common wisdom at the time that amorphization occurs due to the high stresses generated at the shock front. “This led us to ask, is the pre-melting observed in atomistic simulations due to mechanical instabilities, such as strain-induced amorphization, which are not related to melting? Or it is indeed virtual melting? Which loading parameters control the melting? What is the lowest temperature at which melting can occur? These were tough questions,” Levitas acknowledges, “and we then started our collaboration, which after seven years has resulted in the current paper."
Levitas relates the main challenges they faced. “I needed to develop a thermodynamic theory of melting under uniaxial straining typical for shock waves.” At the time, known theories for melting under nonhydrostatic conditions – that is, when loading is different in different directions – considered thermodynamic equilibrium to be between melt and nonhydrostatically stressed solid. Reduction in melting temperature due to nonhydrostatic stresses was estimated to be just 1 °K.
“This definitely did not sound promising,” Levitas continues. “In contrast, melting in our case represents a deformation process and thermodynamic theory for processes was not developed. I did my best to develop a general formal theory and apply it to the specific processes, which Ramon observed in molecular dynamics simulations. “Since Ramon and I have different backgrounds and consider phenomena from completely different positions, one of the most challenging problems was to understand each other and to make our concepts consistent. A few times I misunderstood how Ramon verbally describes results of atomistic simulations, and developed a theory for the wrong scenarios. For example,” Levitas illustrates, “one time I used wrong the video player to play his movie with atomistic simulations and mistakenly observed that melting occurs at the surface of the sample only. I found this very exciting and developed a theory for this scenario, since it sounds reasonable that if uniaxially loaded sample melts along all the surfaces, it would become hydrostatically loaded and further melting would be impossible.”
Their next step, Levitas explains, was determining material parameters for a developed theory and application it to the specific melting process: While thermodynamic theory is quite universal and is applicable to any material, for some materials and loading conditions reduction in melting temperature can be small and for others very large. “This study was done through our synergistic collaboration: Ramon gave me parameters determined from his atomistic simulations for loading of copper and aluminum in several directions. “I then made thermodynamic predictions of the reduction in melting temperature for these specific materials.”
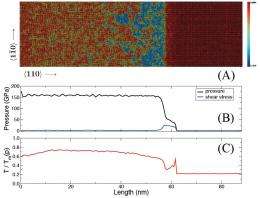
There were also significant challenges in confirming virtual melting by large-scale molecular dynamics simulations. “Thermodynamic theory predicted how much melting temperature can be reduced due to uniaxial versus hydrostatic loading” Levitas explains. “For very high pressure this reduction was drastic: about 10,000 °K. The thermodynamic approach has important advantages in that it’s universal – for example, it’s not limited to specific materials and atomic structure. However, thermodynamics never says that some process will occur, but only that it can occur – that it’s thermodynamically admissible.”
In contrast, Levitas notes, molecular dynamics simulations are performed for a specific material and atomic structure and interaction, but they reproduce all actual physical processes. “It may happen that while melting is possible from the thermodynamic point of view, it does not occur due to kinetic reasons or because other processes – such as dislocation plasticity or twinning – more rapidly release elastic stresses. Thus, one of the challenges in molecular dynamics simulations was to conceptually prove the existence of the virtual melting, which was done for the first time. Another challenge was to prove that the observed disordered state is indeed melt rather than an amorphous solid. Next, we had to investigate which parameters promote the melting, how actual melting temperature depends on them, and identify the lowest melting temperature.”
Addressing these challenges required a range of insights, innovations and techniques. “I believe that the main insights and innovations were in our development of a combined thermodynamic and molecular dynamics approach, as well as in the proof of virtual melting existing in a shock wave several thousand degrees Kelvin below the melting temperature,” Levitas says. “Also, our results further support the idea that the virtual melting is a general phenomenon with various realizations.” While they previously suggested virtual melting as the mechanism of crystal-crystal phase transformation, amorphization and sublimation, here the scientists found that it also can serve as a mechanism of plastic deformation. “Lastly, since the thermodynamic features of the virtual melting here and in previous papers on phase transformations are quite similar, our first molecular dynamics confirmation of the virtual melting indirectly supports the plausibility of the virtual melting as an intermediate stage for phase transformations.”
Levitas outlines how their findings impact nuclear explosions, meteorite impacts, and planned experiments in large laser facilities. “Virtual melting can compete with traditional mechanisms of plastic flow only at very high strain rates. Such conditions can be satisfied during nuclear explosions and meteorite impacts, which is why our results may be utilized for simulation of these phenomena.” Introducing new and completely unexpected deformation mechanisms, he adds, may also lead to essential progress in their understanding and predictive modeling.
“The importance of such high strain rate regimes,” Levitas continues, “is supported by the fact that several laboratories around the world are now developing corresponding facilities for their experimental studies – and our results may find experimental confirmation in such studies. Finally,” Levitas notes, “due to the complexity of interpreting experimental results under such extreme conditions, it’s always good to have an idea about which phenomena could be found – and our virtual melting process is one of them.’
Levitas also describes other areas of research and technology that might benefit from their findings. “In the current paper, virtual melting was conceptually confirmed by molecular dynamics simulations for the most unexpected case of metals, like copper and aluminum.” In these metals, traditional plasticity is very pronounced, which is why extremely high strain rates are required to activate an alternative mechanism like virtual melting. Since thermodynamic consideration is quite generic, this mechanism is expected in many other materials. “For materials with suppressed plasticity – for example, ceramics, high-strength alloys, or complex organic compounds – much lower strain rates may be required,” Levitas points out. “Then the virtual melting may play part in more traditional high-strain rate fields, like penetration of the projectile in a target in armor ceramic, propagation of shock waves, behavior of explosive materials, and deformation in shear bands.”
Regarding next steps in their research, notes Levitas, “We plan to extend our thermodynamic approach for arbitrary 3D loading – in particular for deformation under high pressure and shear strains. It can also be extended for amorphization and sublimation, which can be considered as mechanisms of stress relaxation. Applying a phase field approach to the phenomena discussed is another important task. Finally,” Levitas concludes, “in molecular dynamic simulations we’ll study polycrystalline metals and materials with suppressed plasticity.”
More information: Virtual melting as a new mechanism of stress relaxation under high strain rate loading, PNAS August 14, 2012 vol. 109 no. 33 13204-13207, doi:10.1073/pnas.1203285109
Journal information: Proceedings of the National Academy of Sciences
Copyright 2012 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.