Processes at the surface of catalysts: Oxygen defects act as active centers
In chemical industry, heterogeneous catalysis is of crucial importance to the manufacture of basic or fine chemicals, in catalytic converters of exhaust gas, or for the chemical storage of solar energy. Scientists of Karlsruhe Institute of Technology (KIT) and Ruhr-Universität Bochum (RUB) have developed a new infrared spectroscopy method in order to study processes at surfaces of oxides used as catalysts. Their results are published in the renowned Angewandte Chemie journal.
Catalysts support many chemical reactions. In heterogeneous catalysis, the substance used as a catalyst and the reacting substances exist in various phases. Usually, the catalyst is a solid, while the reacting substances are gaseous. At the surface of catalytically active solids, highly complex chemical processes take place. They have to be understood in detail in order to further improve products and reduce costs. The processes are known well for metals. However, conversions at the surface of oxides – compounds of metals or nonmetals with oxygen – have hardly been studied so far.
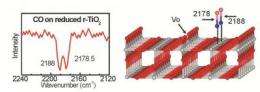
The research team of Professor Christof Wöll from KIT and Professor Martin Muhler from RUB first studied processes at surfaces of oxide monocrystals and then transferred the findings to powders, the technically most important form of oxide materials. Doing this, they were the first to bridge the gap between fundamental research into reference systems and applied research into real catalysts. A newly developed combination device for infrared spectroscopy (IR) allows for highly precise measurements of the vibration frequency of carbon monoxide. The exact value of this vibration frequency is highly sensitive to defects.
Such defects result from the removal of individual oxygen atoms from oxide materials. "Oxygen defects act as active centers and give the material a high catalytic activity," explains Professor Christof Wöll, Director of the Institute of Functional Interfaces (IFG) of KIT. With the new combination device for infrared spectroscopy, the researchers from Karlsruhe and Bochum developed a method that was first calibrated for reference systems. For the first time, they then measured defect densities of real catalyst powders using a high-performance FTIR spectrometer made by Bruker Optics (VERTEX series).
To demonstrate their new method, the researchers used rutile, the most important modification of titanium dioxide (TiO2). "This material used as white pigment and in photocatalysis normally is chemically highly inert and rendered catalytically active by the oxygen defects only," explains Professor Christof Wöll. Professor Martin Muhler from RUB points out that such defects in powder materials have only been detected indirectly so far.
With their method, the researchers, including Dr. Mingchun Xu, Dr. Heshmat Noei, and Dr. Yuemin Wang from RUB as well as Dr. Karin Fink from the Institute of Nanotechnology (INT) of KIT, followed the "Surface Science" approach developed by the Noble Prize laureate Gerhard Ertl. They demonstrated the potential of their method by studying the carbon-carbon coupling reaction of formaldehyde to ethylene. Doing this, it was confirmed that the density of oxygen defects at the surface of r-TiO2 nanoparticles is of decisive im-portance to the catalytic activity of the oxide powder and, hence, to the yield.
More information: Mingchun Xu, Heshmat Noei, Karin Fink, Martin Muhler, Yuemin Wang, and Christof Wöll. The Surface Science Approach for Understanding Reactions on Oxide Powders: The Importance of IR Spectroscopy. Angewandte Chemie, published online on April 5, 2012. DOI: 10.1002/ange.201200585
Journal information: Angewandte Chemie
Provided by Helmholtz Association of German Research Centres



















