When cells divide
For two independent daughter cells to emerge from a cell division, the membrane of the dividing cell must be severed. In the latest issue of Science, a team led by Daniel Gerlich, Professor at the Institute of Biochemistry at ETH Zurich, presents a model illustrating this last step in the division of human cells.
Before a cell divides, all the essential components, for example the genome, are duplicated. The DNA segregates to the two daughter cells by migrating along tube-like structures called microtubules. In the meantime, a contractile protein ring mediates progressive narrowing of a cleavage furrow. This finally leads to the formation of a thin intercellular canal, which is the only remaining connection between the daughter cells. At this stage, the microtubules along which the DNA previously migrated to the opposite poles of the cell, form a dense bundle within this intercellular canal. To complete the cell division, the microtubules must be disassembled and the cell membrane severed to form two independent daughter cells. A team led by Daniel Gerlich, Professor at the Institute of Biochemistry at ETH Zurich, and Thomas Müller-Reichert from Dresden University of Technology has researched this final step in cell division. What exactly happens when the cell membrane divides and the microtubules disassemble?
A challenge at small scales
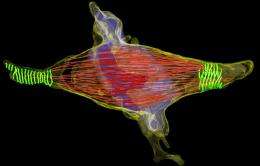
Finding the answer to this question is not easy: the intercellular canal is a tiny structure with a diameter of only one thousandth of a millimeter. This and the fact that the intercellular canal disassembles during a short period of only five to ten minutes makes it difficult to study the underlying processes. The scientists led by Gerlich used a light microscope to observe living human cells in the late stage of cell division. The dismantling of the microtubules in the intercellular canal indicated that separation of the cells was about to occur. The researchers then immediately preserved the cells chemically and cut them into thin slices for high-resolution imaging using an electron microscope. By this "correlative imaging" approach, they discovered that the surface of the intercellular canal showed a regularly rippled structure.
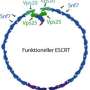
Further studies using three-dimensional electron microscopy showed that the crinkled structure was made up of thread-like proteins (filaments) that constricted the intercellular bridge in a spiral arrangement. This has a certain similarity with the protein ring that increasingly narrows down the cleavage furrow at an earlier stage in cell division. Therefore the scientists speculated that these filaments, which they had newly discovered, could exert a force on the intercellular canal that could constrict and ultimately sever the membrane.
The missing link
Earlier studies had shown that the microtubules in the intercellular canal are disassembled by the enzyme spastin. However, the results of the research by Gerlich and Reichert indicated that the activity of spastin alone is insufficient to separate the daughter cells. Therefore the scientists speculated that the newly discovered protein filaments could be the missing link that is indispensable for the separation of the intercellular canal. They suspected that the newly discovered protein filaments might be constructed from the protein complex ESCRT III. ESCRT III is an enzyme that helps to pinch off membranes from the cell surface, e.g. when viruses exit from an infected cell. This property of ESCRT III renders it a possible candidate for helping to sever the connection between daughter cells. The scientists showed that ESCRT III does indeed accumulate in the intercellular bridge shortly before the membrane divides. If they removed ESCRT III, no filament spirals formed on the surface of the intercellular bridge, and the separation between the cells could not take place. Other studies had previously shown that under certain conditions, ESCRT III can indeed form long protein chains. Together, these observations suggest that the contractile structure at the intercellular bridge could consist of ESCRT III protein filaments.
Open questions
Definitive proof that the newly discovered filaments are indeed made of ESCRT III, however, is still lacking. The mechanism by which the filaments constrict the intercellular bridge between the daughter cells and cut the membranes apart also remains unclear. Gerlich says, “We are excited to address these important questions.” Through their research results, the scientists led by Gerlich and Reichert have developed a new and convincing model for the severing of the connection between daughter cells: An ESCRT III-mediated constriction of the intracellular canal and simultaneous disassembly of microtubules inside by spastin lead to the formation of two independent daughter cells.
More information: Guizetti J, et al. Cortical Constriction During Abscission Involves Helices of ESCRT-III–Dependent Filaments. Science 25 March 2011: Vol. 331 no. 6024 pp. 1616-1620. DOI: 10.1126/science.1201847
Provided by ETH Zurich