Long and narrow, free of defects, and soluble: graphene nanoribbons by bottom-up synthesis
(PhysOrg.com) -- Electronic components based on graphene could render our current silicon-based electronics obsolete. Graphene, a more recently discovered form of carbon, consists of two-dimensional sheets of aromatic six-membered carbon rings in a honeycomb arrangement. In contrast to extended graphene layers, narrow graphene nanoribbons have semiconducting properties and could thus be candidates for electronic applications.
Klaus Müllen and a team from the Max Planck Institute for Polymer Research in Mainz have now introduced a new method for the synthesis of long, narrow graphene ribbons with defined dimensions in the journal Angewandte Chemie.
Previously, graphene ribbons were mainly cut out of larger graphene sheets or were obtained by slitting open carbon nanotubes lengthwise. However, with these methods it is impossible to produce ribbons that have a precisely established ratio of width to length as well as defined edges. These details are important because they determine the electronic properties of the ribbons. The search has thus been on for a method that allows controlled production of very narrow graphene ribbons—an extremely difficult challenge. The German researchers working with Müllen are now well on the way to overcome it. They are not starting with large structures to cut up (top-down); instead they are building their ribbons from smaller components (bottom-up).
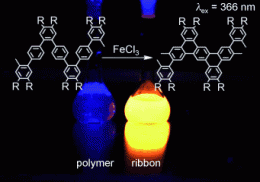
The building blocks selected by Müllen and his team are long chains of aromatic six-membered carbon rings called polyphenlyenes. In contrast to previous approaches, they did not produce straight chains; instead they made them with a flexible, zigzagging, bent backbone. Furthermore, they attached hydrocarbon side-chains to the backbone to increase the solubility in organic solvents, which allows the compounds to be synthesized and processed in solution.
The next step is a reaction that splits off hydrogen (dehydrogenation). This causes a ring-closing reaction in each pointy tip of the zigzag, forming a new aromatic six-membered carbon ring that shares a side with three neighboring rings—the chain transforms in to a narrow ribbon.
In this way, the team was able to produce a series of different nanoribbons with lengths reaching over 40 nm. The width of the ribbon was defined by the size of the “points” of the polyphenylene precursor. The resulting ribbons are free of defects and soluble in common organic solvents.
“We have been the first to demonstrate that structural perfection can be achieved by the classical bottom-up synthesis of defined graphene nanoribbons,” says Müllen. “The solubility of the ribbons is an important requirement for the large-scale production of electronic components.”
More information: Klaus Müllen, Graphene Nanoribbons by Chemists: Nanometer-Sized, Soluble, and Defect-Free, Angewandte Chemie International Edition, Permalink to the article: dx.doi.org/10.1002/anie.201006593
Provided by Wiley


















