Little Fish, Big Science

(PhysOrg.com) -- The zebrafish population at Duke is about to boom. It turns out those puny, striped fish (they get their name honestly) that dart around many a household aquarium are ideal specimens for unraveling disease. New Duke faculty member Nico Katsanis thinks that zebrafish may hold the key to tackling some of the biggest obstacles facing genome scientists today.
The challenge is this: genome technologies have made it a snap to generate sequence data and identify spots in the genome where one person might differ from the next. But the ease of sequencing has now far outstripped scientists skill in identifying medically important variations. As a result, researchers sometimes get stuck “data gazing.” Or they may focus in too early on certain genes based on what they already know or think they know, potentially missing big or unexpected discoveries in the process.
“I think that can be dangerous, because you no longer look at the big picture but instead use a narrow genetic model to try to sort out which mutations might be relevant to the disease or trait you study,” Katsanis says.
As director of the Center for Human Disease Modeling, a new School of Medicine entity, Katsanis intends to narrow down the hundreds of potential leads that genome studies can turn up to a short list of prime suspects in a less biased way. He likes to think of his new center as an “an intellectual hotel” where people can come to mull over and work together to solve problems they have found intractable by other means.
“Nico’s combination of expertise in human genetics and zebrafish is unique and he is a very interactive person,” said Ken Poss, an associate professor of cell biology and fellow zebrafish guru. “I think he will make things happen here.”
Katsanis is drawn to challenges. In graduate school he mapped and cloned candidate genes for Down’s syndrome, but ultimately found the work too mainstream for his taste. When his postdoctoral advisor asked him to tackle a virtually unknown and hard-to-crack disease - Bardet-Biedl Syndrome (BBS) - Katsanis jumped at the chance. BBS is a rare genetic disorder that affects a variety of different organ systems, resulting in obesity, visual impairment, mental retardation and kidney problems. It took what Katsanis described as an “atrocious” two and a half years before they uncovered the first gene for the disease.
“Every hypothesis I had about the syndrome was wrong, but the truth was more exciting,” said Katsanis. “It has taught me to try as hard as I can not to pigeon-hole myself and to just let the science take me where it takes me. I have to tell you, it has been a hell of a ride.”
Lately, the science has led him to analyze every single disease variant reported in BBS patients - about 150 in all - and examine their function in zebrafish.The results have been surprising. In twenty percent of BBS patients, it takes a combination of three mutations to manifest the disease.
“We have had blinders on for a long time, but I now know that if you take those blinders off and look at the data objectively you come up with new ideas,” said Katsanis. “We have a far more precise - not fully accurate - but far more precise notion of the disease architecture from doing this model-free experiment. And I am willing to bet - in fact, I am betting my entire career - that this is going to be an approach that will be useful for many other problems of clinical relevance.”
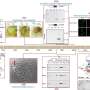
Katsanis' new center can handle organisms from worms to flies, but he chooses to focus his own efforts on zebrafish, whose transparent bodies make it easy to observe their inner workings as they develop - craniofacial malformations, heart defects, aberrant signaling pathways and all. Because zebrafish share 70 percent of their genes with humans, they can also be “humanized,” meaning that the fish version of a gene or set of genes can be replaced by their human equivalents.
Katsanis’ team uses specially designed strings of nucleotide bases - As, Ts, Cs and Gs - to mask specific gene messages in zebrafish embryos. At the same time, they insert a normal or mutated human version of the gene. In that way, they can see how those human variants affect development - this fish is growing a tail, this one is not; this fish is making a cortex, that one is not. They can even watch as molecular-level events unfold in real time. The center expects to generate zebrafish with hundreds to thousands of gene variants per month.
Ultimately, Katsanis hopes to encourage a shift in the approach to complex disease all the way down to the level of personal genomes.
“I think that in the future, we should be able to gaze at someone’s genome and make a pretty strong argument about what is going to happen and what is going to be helpful to them,” said Katsanis. “I am sure that you or I have random mutations throughout our genomes, but they couldhave nothing to do with any medical problems that you or I develop. The question is what variation is relevant to the problems you are interested in.”
Provided by Duke University