Scientists use high-pressure 'alchemy' to create nonexpanding metals
By squeezing a typical metal alloy at pressures hundreds of thousands of times greater than normal atmospheric pressure, scientists at the California Institute of Technology (Caltech) have created a material that does not expand when heated, as does nearly every normal metal, and acts like a metal with an entirely different chemical composition.
The discovery, described in a paper in Physical Review Letters (PRL), offers insight into the exotic behavior of materials existing at high pressures—which represent some 90 percent of the matter in our solar system.
Zero-expanding metal alloys were discovered in 1896 by Swiss physicist Charles Édouard Guillaume, who worked at the International Bureau of Weights and Measures in France. While attempting to develop an inexpensive international standard for the meter, the metric unit of length, Guillaume hit upon an inexpensive iron-nickel alloy that expands very little when heated. He dubbed the material an "Invar" alloy—because the metals are "invariant" when heated, such that the length of a piece of Invar metal does not change as its temperature is increased, as do normal metals. Since Guillaume's discovery—which, in 1920, earned him the Nobel Prize in Physics (besting Albert Einstein, who was awarded the prize in 1921)—other nonexpanding alloys have been identified.
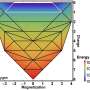
It has long been known that Invar behavior is caused by unusual changes in the magnetic properties of the alloys that somehow cancel out the thermal expansion of the material. (Normally, heat increases the vibrations of the atoms that make up a material, and the atoms prefer to move apart a little, causing expansion.)
"Recent computer simulations indicate that electrons in Invar alloys take on a special energy configuration," says Caltech graduate student Michael Winterrose, the first author of the PRL paper. "This energy state is at the borderline between two types of magnetic behavior, and is very sensitive to the precise ratio of elements that make up the alloy. If you move away from the Invar chemical composition by only a couple of percent, the energy configuration will disappear," he says.
Because of their unresponsiveness to temperature change, Invar alloys have been used in devices ranging from watches, toasters, light bulbs, and engine parts to computer and television screens, satellites, lasers, and scientific instruments. "In our day-to-day lives, we are surrounded by items that make essential use of Invar alloys," Winterrose says.
The Caltech scientists did not set out to study Invar behavior—and, in fact, were hoping to avoid it. "We intentionally picked chemical compositions that do not show Invar behavior because I thought it would confuse our interpretations," says Brent Fultz, a professor of materials science and applied physics at Caltech, and a coauthor of the PRL paper.
Instead, Winterrose, Fultz, and their colleagues were examining the effect of pressure on the alloy of palladium (Pd) and iron (Fe) called Pd3Fe, where three of every four atoms are palladium, and one is an iron atom. (In the similarly named but chemically distinct PdFe3—which is a traditional Invar alloy—three of every four atoms are iron, and one is palladium).
"The Fe and Pd atoms [in the alloy] have very different sizes, and we expected to see some interesting effects from this size difference when we put Pd3Fe under pressure and measured its volume," Winterrose explains. To test this, the scientists squeezed a small sample of the material between two diamond anvils, generating pressures inside the sample that were 326,000 times greater than standard atmospheric pressure.
"Our initial results from these studies showed that the alloy stiffened under pressure, but far more than we expected," he says. To figure out the cause, the scientists simulated the quantum mechanical behavior of the electrons in the alloy under pressure. "The simulations showed that under pressure, the electrons found the special energy levels between strong and weak magnetism that are associated with normal Invar behavior. Up to this point we had been quite unaware of the possibility for Invar behavior in our material," Winterrose says.
Subsequent experiments at the Advanced Photon Source at Argonne National Laboratory in Chicago and the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory in New York confirmed that the intense pressure had indeed suppressed thermal expansion in Pd3Fe, much like tuning the chemical composition.
The scientists had performed a kind of high-pressure "alchemy" on the alloy, where pressure makes the electrons act as if they are around atoms of a different chemical element, Winterrose says.
The research helps unify our understanding of Invar behavior, which is one of the oldest and most-studied unresolved problems in materials research. In addition, using pressure to force electrons into new states can point to directions in materials chemistry where new properties can be found, at least for magnetism.
"Today, materials physics has some excellent computational tools for predicting the structure and properties of materials, although there are suspicions about how well they work for magnetic materials," says Fultz. "It is satisfying that these computational tools worked so well for showing how pressure changed the material into an Invar alloy. Invar behavior is pretty subtle, requiring a very special condition for the electrons in the metal that is usually tuned by precise control of chemical composition. Pressure can make the electrons behave as if they are in a material of different chemical composition, so I really like Mike's use of the word 'alchemy'."
Source: California Institute of Technology (news : web)