Batteries get a (nano)boost
Need to store electricity more efficiently? Put it behind bars. That's essentially the finding of a team of Rice University researchers who have created hybrid carbon nanotube metal oxide arrays as electrode material that may improve the performance of lithium-ion batteries.
With battery technology high on the list of priorities in a world demanding electric cars and gadgets that last longer between charges, such innovations are key to the future. Electrochemical capacitors and fuel cells would also benefit, the researchers said.
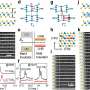
The team from Pulickel Ajayan's research group published a paper this week describing the proof-of-concept research in which nanotubes are grown to look - and act - like the coaxial conducting lines used in cables. The coax tubes consist of a manganese oxide shell and a highly conductive nanotube core.
"It's a nice bit of nanoscale engineering," said Ajayan, Rice's Benjamin M. and Mary Greenwood Anderson Professor in Mechanical Engineering and Materials Science.
"We've put in two materials - the nanotube, which is highly electrically conducting and can also absorb lithium, and the manganese oxide, which has very high capacity but poor electrical conductivity," said Arava Leela Mohana Reddy, a Rice postdoc researcher. "But when you combine them, you get something interesting."
That would be the ability to hold a lot of juice and transmit it efficiently. The researchers expect the number of charge/discharge cycles such batteries can handle will be greatly enhanced, even with a larger capacity.
"Although the combination of these materials has been studied as a composite electrode by several research groups, it's the coaxial cable design of these materials that offers improved performance as electrodes for lithium batteries," said Ajayan.
"At this point, we're trying to engineer and modify the structures to get the best performance," said Manikoth Shaijumon, also a Rice postdoc. The microscopic nanotubes, only a few nanometers across, can be bundled into any number of configurations. Future batteries may be thin and flexible. "And the whole idea can be transferred to a large scale as well. It is very manufacturable," Shaijumon said.
The hybrid nanocables grown in a Rice-developed process could also eliminate the need for binders, materials used in current batteries that hold the elements together but hinder their conductivity.
The paper was written by Reddy, Shaijumon, doctoral student Sanketh Gowda and Ajayan. It appears in the online version of the American Chemical Society's Nano Letters.
Source: Rice University