December 2, 2008 feature
A Promising Catalyst for Solar-Based Hydrogen Energy Production
(PhysOrg.com) -- Scientists have found that a polymer material is an excellent catalyst in a process to produce hydrogen fuel using sunlight and water. The material meets the basic requirements for an ideal catalyst -- including being abundant, easy to work with, and non-toxic -- and could help this "green" alternative-energy production method become mainstream.
Creating hydrogen gas by splitting water (H2O) molecules with solar energy is a promising way of generating hydrogen fuel, which, by either being burned directly or used in fuel cells, can power many types of vehicles, including automobiles, buses, and even airplanes.
The study's corresponding scientist is Xinchen Wang, a chemist affiliated with the Max-Planck Institute of Colloids and Interfaces in Potsdam, Germany, and Fouzhou University in Fouzhou, China.
"The search for a suitable semiconductor material to use as a catalyst in this process has been a main goal of materials-science research," said Wang to PhysOrg.com. "In addition to being a plentiful, versatile, and safe material, the catalyst should also be stable when in contact with water and able to absorb visible light. The material we chose fits these requirements."
The inorganic catalysts developed in the last 30 years have been metal-based and often require the use of pricey precious metals to aid in the catalysis process. Synthetic polymer materials have also been developed, but they only work with light in the ultraviolet region -- a small fraction of the solar spectrum -- and their performance is mediocre.
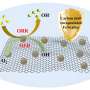
The material investigated by Wang and his colleagues is carbon nitride that has been "polymerized" into molecule chains. This form of carbon nitride was first synthesized in 1834. The group went a step further, using a heating/condensation process to force the chains to form layered sheets with structures similar to graphite, a highly ordered form of carbon.
The carbon nitride was then powdered and added to water containing a "reagent" material that donates electrons to the catalysis reaction. When the mixture is illuminated, the water molecules split into positive hydrogen ions and oxygen atoms. The catalyst's carbon atoms assist by providing locations for the hydrogen-ions to reduce to H2 -- a process by which the nitrogen atoms "donate" electrons to the ions so they can reform into diatomic hydrogen. The nitrogen atoms help with the opposite process, oxidation, helping the oxygen atoms form O2 molecules.
The group's tests show that polymeric carbon nitride absorbs both ultraviolet and visible light and, although its performance yielded varying H2 production rates from batch to batch, that it is an effective catalyst even without the presence of platinum or other noble metals.
"Our result opens new pathways for the search of energy production schemes, using polymeric organic semiconductor structures that are cheap, stable, and commonly available," said Wang.
This study is published in the November 9 online edition of Nature Materials.
More information: Xinchen Wang, Kazuhiko Maeda, Arne Thomas, Kazuhiro Takanabe, Gang Xin, Johan M. Carlsson, Kazunari Domen & Markus Antonietti, Nature advance online publication DOI:10.1038/nmat2317
Copyright 2007 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.