Switchable Lotus Effect
Lotus blossoms are beautiful, and always immaculately clean. Water drops bead up and roll off of their water-repellent surface, washing away every speck of dust. This type of self-cleaning surface would be very useful to us as well: no more carwash, no soiled facades on houses—the potential uses are endless. To date, however, technology has not been able to duplicate nature’s success.
Researchers led by Kingo Uchida and Shinichiro Nakamura have now synthesized a compound in the diarylethene family whose surface becomes super-water-repellent on command.
The secret behind the lotus effect is the special microstructure, consisting of tiny nubs, on the surface of the lotus plant’s leaves. These micronodules provide no surface on which water drops can collect, so the leaf does not get coated with water. The drops contract into beads and roll off the surface, sweeping away any particles of dirt they encounter on the way. On normal smooth surfaces, water drops coat the surface and assume a hemispherical shape. Instead of rolling, they then glide over the surface, which does not allow them to remove dirt particles.
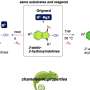
The Japanese researchers have now synthesized a special substance, a member of the group of compounds known as diarylethenes, and produced a microcrystalline film of this substance on a support. Electron microscopy images show that the surface of this film is initially smooth. When the diarylethene film is irradiated with UV light, the previously colorless surface turns blue—and is no longer smooth.
Instead it is covered with a fine down of tiny fibers that have a diameter of about 1 µm. This down has a similar effect to the micronodules on the lotus blossom, resulting in a super-water-repellent surface. If the surface is irradiated again, this time with visible light, the fibers and color vanish, leaving a colorless, smooth, and wettable surface.
This effect originates from changes in the molecular structure. The diarylethene molecule is made of three five-membered rings hooked together. UV light sets off a rearrangement within the molecule (isomerization). This results in a ring closure, which leads to formation of a fourth ring. The isomer with the closed fourth ring crystallizes in the form of needles, which grow out of the crystals of the isomer with the open ring as soon as a certain concentration is reached. Light in the visible range of the spectrum sets off the reverse reaction: the ring re-opens, and the needles disappear.
Citation: Kingo Uchida et al., "Photoinduced Reversible Formation of Microfibrils on a Photochromic Diarylethene Microcrystalline Surface", Angewandte Chemie International Edition, doi: 10.1002/anie.200602126
Source: Angewandte Chemie