Watch amazing footage of how nanotubes form
A team of scientists led by the Department's Dr Stephan Hofmann have successfully produced live video footage that shows how carbon nanotubes, more than 10,000 times smaller in diameter than a human hair, form.
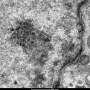
The video sequences can be viewed by clicking the links below. They show nanofibres and nanotubes nucleating around miniscule particles of nickel and are already offering greater insight into how these microscopic structures self-assemble.
These two videos show how the nickel reacts a process called catalytic chemical vapour deposition (CVD). This is one of several methods of producing nanotubes, and involves the application of a gas containing carbon (in this case acetylene) to minute crystalline droplets referred to as "catalyst islands" (the nickel).
In conditions appropriate to creating nano-fibres, the catalyst was squeezed upwards gradually as carbon formed around it. When the application of gas was reduced to create single-walled nanotubes, the carbon instead lifted off the catalyst to form a tubular structure.
In particular, the team discovered that the carbon network is guided into tubular shape by a drastic restructuring of the nickel – the catalyst in the process. They were also able to track and time the deposition of the carbon around the nickel.
Carbon nanotubes are new building blocks enabling engineers to improve and further miniaturise everyday electronic devices like computers or mobile phones. At the moment scientists can grow nanotubes but cannot accurately control their structure.
Being able to do so is vital as it is the very structure of a nanotube that dictates its properties. The nano-scale video observations mean that scientists will be able to better understand the nucleation of nanotubes and are therefore an important step on the route towards application.
The two sequences show action taking place in real time on an astonishingly small scale. The difference in size between a single-walled nanotube and a human hair is close to the difference between the same human hair and the Eiffel Tower. The microscopic scale involved has, in the past, made it difficult to understand the growth process.
The team used X-rays produced at a synchrotron (a type of particle accelerator) and a modified high-resolution transmission electron microscope to observe and film the catalytic chemical vapour deposition process.
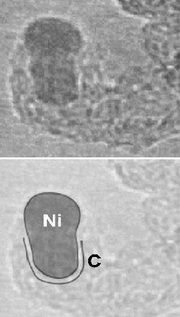
As the gas is applied carbon sticks to the catalyst islands forming layers of graphite. In conditions appropriate to creating nanofibres, the nickel particle was pushed upwards in a series of peristaltic movements as the carbon continued to deposit on its sides. At several points the nickel formed a cap which almost “popped” out of the forming tube, leaving a layer of graphite behind it. This process is called “bambooing”, because the resultant carbon nanofibre is a cylinder containing several cavities, each one separated by one of these graphite layers, similar in form to bamboo. Throughout the whole process, the nickel remained crystalline rather than liquid.
The team then looked at conditions more appropriate to producing single-walled carbon nanotubes, which involved less acetylene. The catalyst is not squeezed upwards. Instead, a cap of carbon formed on the top of the nickel, and gradually extended from it to form a tubular structure. The catalyst island was squeezed and reshaped by this process and was moulded by the carbon forming around it rather than retaining its original form.
Dr Stephan Hofmann, who led the research, said: “In order to reach the full application potential for nanotubes, we need to be able to accurately control their growth first. As a manifestation of the impressive progress of nanometrology, we are actually now able to watch molecular objects grow. This new video footage shows that the catalyst itself remains crystalline but is constantly changing its shape as the carbon network is formed around it.
“We cannot yet solve the problem of not being able to self-assemble carbon nanotubes with well-defined characteristics, but we have discovered that if we are to do so, we need to be mindful not just of the carbon dynamics but the changing shape of the catalyst as well.”
Source: University of Cambridge