Improved Self-Assembly of Nanomaterials May Enhance Solar Cells

Novel, self-assembly techniques for fabricating inorganic nanomaterials that could pave the way for more efficient and powerful solar cells, chemical sensors and detectors currently are being developed by a University at Buffalo chemist.
David F. Watson, Ph.D., an assistant professor in the Department of Chemistry in the University at Buffalo's College of Arts and Sciences, has been awarded a prestigious National Science Foundation CAREER Award to conduct the research.
According to the NSF, the CAREER program recognizes and supports the early career-development activities of teacher-scholars "who are most likely to become the academic leaders of the 21st century."
The research component of the grant involves a new approach to photochemistry, chemical reactions involving light, while the educational component will introduce students in the Buffalo Public Schools from underrepresented groups, including Native Americans, to principles of materials chemistry and scientific research through hands-on science activities.
The grant, which provides $576,100 over five years, will allow Watson and colleagues to conduct research aimed at better controlling the electron transfer reactivity of self-assembled inorganic nanomaterials.
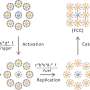
In particular, Watson's group is studying and characterizing photo-induced surface electron transfer reactions occurring within self-assembled inorganic nanomaterials, the reactions that drive solar cells and photocatalysts. The scientists will continue work on a self-assembly technique Watson developed for attaching quantum dots, tiny light-absorbing particles, to metal oxide films.
Using time-resolved spectroscopy, the researchers are able to probe systematically how composition, morphology and physical properties of the materials affect the kinetics and efficiency of electron transfer processes.
The researchers also will study how to improve the targeted patterning of nanoparticles onto metal oxide surfaces.
"This photochemical patterning strategy addresses one of the significant challenges in nanofabrication, to control both short-range and long-range order in nanostructured materials," said Watson.
Short-range order refers to the organization of molecules and materials on the nanometer scale, while long-range order involves pattern formation on larger, even macroscopic, dimensions.
Watson's approach combines the "top-down" and "bottom-up" methods of fabricating nanomaterials into a hybrid technique, in which photochemical reactions are used to organize nanoparticles on surfaces.
Substrates with high surface areas, he explained, allow for optically dense patterns and more efficient light harvesting, thereby potentially increasing the efficiency of solar cells and other devices.
"Because our surface substrate is the photochemically active component, our approach also might enable more widely applicable patterning techiques," he said.
Watson's grant also will provide summer research internships to students at various high schools in Buffalo through collaborations with faculty in the departments of chemistry and physics in the UB College of Arts and Sciences and in the departments of chemical and biological engineering and electrical engineering in the UB School of Engineering and Applied Sciences.
The educational program builds on the extensive partnership that exists between UB's Department of Chemistry and Buffalo Public School 19, a Native American magnet school for middle school students.
Also with the support of the CAREER award, Watson is designing a "writing-intensive" course for advanced undergraduates and graduate students in the Department of Chemistry that will address one of his key educational concerns.
"Chemistry majors typically don't do a lot of writing during their undergraduate or graduate careers, but it's a huge part of what we do as scientists," he said. "The idea is to get the students used to doing a lot of writing and to write mock reviews and critique each others' work."
Source: University at Buffalo