Researchers find controls to gold nanocatalysis
Researchers at the Georgia Institute of Technology have made a discovery that could allow scientists to exercise more control over the catalytic activity of gold nanoclusters.
The finding – that the dimensionality and structure, and thus the catalytic activity, of gold nanoclusters changes as the thickness of their supporting metal-oxide films is varied – is an important one in the rapidly developing field of nanotechnology. This and further advances in nanocatalysis may lead to lowering the cost of manufacturing materials from plastics to fertilizers.
The research appeared in the July 21, 2006 issue of the journal Physical Review Letters.
"We've been searching for methods for controlling and tuning the nanocatalytic activity of gold nanoclusters," said Uzi Landman, director of the Center for Computational Materials Science and Regents' professor and Callaway chair of physics at Georgia Tech. "I believe the effect we discovered, whereby the structure and dimensionality of supported gold nanoclusters can be influenced and varied by the thickness of the underlying magnesium-oxide film may open new avenues for controlled nanocatalytic activity," he said.
Landman's research group has been exploring the catalytic properties of gold, which is inert in its bulk form, for about seven years. In 1999, along with the experimental group of Ueli Heiz and Wolf-Dieter Schneider at the University of Lausanne, Landman's group showed that gold exhibits remarkable catalytic capabilities to speed the rate of chemical reactions if it is clustered in groups of eight to about two dozen atoms in size.
Last year in the journal Science, the teams of Landman and Heiz (now at the Technical University of Munich) showed that this catalytic activity involves defects, in the form of missing oxygen atoms, in the catalytic bed on which the gold clusters rest. These defect sites, referred to as F-centers, serve as sites for the gold to anchor itself, giving the gold clusters a slight negative charge. The charged gold transfers an electron to the reacting molecules, weakening the chemical bonds that keep them together. Once the bond is sufficiently weakened, it may be broken, allowing reactions to occur between the adsorbed reactants.
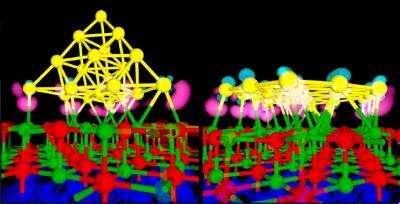
Now Landman's group has found that by using a thin catalytic bed with a thickness of up to 1 nanometer (nm), or 4-5 layers, of magnesium oxide, one may activate the gold nanoclusters which may act then as catalysts even if the bed is defect-free. A model reaction tested in these studies is one where carbon monoxide and molecular oxygen combine to form carbon dioxide, even at low temperatures. In these reactions, the bond connecting the two atoms in the adsorbed oxygen molecule weakens, thus, promoting the reaction with CO.
In this study, Landman and company simulated the behavior of gold nanoclusters containing eight, sixteen and twenty atoms when placed on catalytic beds of magnesium oxide with a molybdenum substrate supporting the magnesium oxide film. Quantum mechanical calculations showed that when the magnesium oxide film was greater than 5 layers or 1 nm in thickness, the gold cluster kept its three-dimensional structure. However, when the film was less than 1nm, the cluster changed its structure and lied flat on the magnesia bed –wetting and adhering to it.
The gold flattens because the electronic charge from the molybdenum penetrates through the thin layer of magnesium oxide and accumulates at the region where the gold cluster is anchored to the magnesium oxide. With a negative charge underneath the gold nanocluster, its attraction to the molybdenum substrate, located under the magnesia film, causes the cluster to collapse.
"It's the charge that controls the adhesive strength of gold to the magnesia film, and at the same time it makes gold catalytically active," said Landman. "When you have a sufficiently thin layer of magnesium oxide, the charge from the underlying metal penetrates through – all the way to the interface of the gold cluster."
In the previous experimental studies, defects in the magnesium oxide were required to bring about charging of the adsorbed clusters.
"Until now, the metal substrate was regarded only as an experimental necessity for growing the magnesium oxide films on top of it. Now we found that it can be used as a design feature of the catalytic system. This field holds many surprises," said Landman.
Landman's group is currently undertaking further explorations into possibilities to regulate the charge, and hence the catalytic activity, in gold nanocatalytic systems.
Landman and Heiz's book titled "Nanocatalysis" is scheduled to be published this month.
The current research was performed at the Center for Computational Materials Science by postdoctoral fellows Davide Ricci and Angelo Bongiorno under the supervision of Landman. The research team also included Dr. Gianfranco Pacchioni, a colleague from the University of Milano.
Source: Georgia Institute of Technology





















