Carbon nanotubes offer 'green' technology for perchlorate removal
Researchers at Pacific Northwest National Laboratory have demonstrated a new, environmentally friendly process for treating water contaminated by perchlorate, a toxic chemical that has been found in drinking water in 35 states.
Perchlorate is used in rocket fuel, fireworks and defense manufacturing, and groundwater contaminated by the chemical is difficult to treat. High levels of perchlorate have been associated with thyroid disease, plus the possibility of cancer and other health problems. Contamination is especially widespread in California, where there are many U.S. military bases.
The conventional method for treating perchlorate-contaminated water employs an ion exchange resin. Regenerating the resin requires flushing with an acidic solution, which results in large quantities of secondary waste.
The PNNL method is an electrically controlled anion exchange process. “The technology is unique in that it uses an electric current to regenerate the resin and release the perchlorate without producing a lot of secondary waste,” said Yuehe Lin, lead scientist for the research, adding that the process is “green” because it produces so little waste.
The technology is available for licensing and joint research opportunities through Battelle, which operates PNNL for the Department of Energy and facilitates the transfer of lab-created technologies to the marketplace.
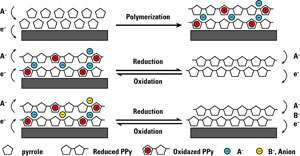
To create the new process, Lin and his colleagues induced a positive charge to an electrically conducting polymer, such as a polypyrrole, that selectively attracts the negatively charged perchlorate ions. Application of an electric current releases the trapped perchlorate ions for disposal. Now neutral, the polymer can be reverted to a positively charged surface and re-used.
The scientists increased the amount of perchlorate that can be captured by depositing the polymer as a polypyrrole thin film on a matrix of carbon nanotubes, creating a porous conductive nanocomposite.
“The high surface area of the carbon nanotubes provides an ideal matrix for the polymer,” Lin said, noting that the polymer is electrodeposited on the carbon nanotubes through in situ polymerization.
The porous surface created by the carbon nanotubes also gives the technology a longer life cycle because the polymer is more stable on the nanotube matrix than it would be on a flat, conducting substrate.
The electrically controlled anion exchange technology can be used to remove other contaminants, such as cesium and chromium. A radioactive material common to nuclear waste sites, cesium could be used by terrorists to build dirty bombs or contaminate drinking water. Chromate is a toxic form of chromium that is readily absorbed by the body.
Source: PNNL




















