Method slashes quantum dot costs by 80 percent
Rice scientists replace pricey solvents with cheap processing fluids
In an important advance toward the large-scale manufacture of fluorescent quantum dots, scientists at Rice University have developed a new method of replacing the pricey solvents used in quantum dot synthesis with cheaper oils that are commonplace at industrial chemical plants.
Rice's study, which was conducted under the auspices of the Center for Biological and Environmental Nanotechnology (CBEN), is published online and slated to appear in the October issue of the journal Nanotechnology.
"CBEN started to undertake some exploratory work more than a year ago on the scale-up issues of quantum dot manufacture, but the solvents turned out to be so expensive that we just couldn't afford to run more than a few large-reactor experiments," said the study's lead author, Michael Wong, assistant professor of chemical and biomolecular engineering and of chemistry. "That was a great reality check, and it made us look at the problem of solvent cost sooner rather than later."
Quantum dots typically cost more than $2,000 per gram from commercial sources, and pricey solvents like octadecene, or ODE - the least expensive solvent used in quantum dot preparation today - account for about 90 percent costs of raw materials.
Heat-transfer fluids - stable, heat-resistant oils that are used to move heat between processing units at chemical plants - can cost up to seven times less than ODE. Replacing ODE with the heat-transfer fluid Dowtherm A, for example, reduces the overall materials cost of making quantum dots by about 80 percent.
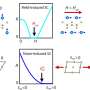
Quantum dots are tiny crystals of semiconducting materials - cadmium selenide or CdSe is the most popular flavor - that measure just a few nanometers in diameter. Most of the commercial possibilities discussed for quantum dots - bioimaging, color displays, lasers, etc. - relate to their size-controlled fluorescence. For example, CdSe quantum dots have the ability to absorb high-energy photons of ultraviolet light and re-emit them as photons of visible light. They glow different colors, depending on the size, shifting from the red to the blue end of the spectrum as the crystals get smaller.
The reproducible synthesis of high-quality quantum dots became a reality in the early 1990s when researchers at MIT pioneered a new method of producing quantum dots with uniform sizes and well-defined optical signatures. The basic recipe for making quantum dots hasn't changed much since it was first developed. A solvent is heated to almost 500 degrees Fahrenheit, and solutions containing cadmium and selenium compounds are injected. They chemically decompose and recombine as pure CdSe nanoparticles. Once these nanocrystals form, scientists can adjust their optical properties by growing them to precisely the size they want by adjusting the cooking time.
The solvent originally used for this process was trioctylphosphine oxide, or TOPO, which costs more than $150 per liter. Later, other scientists introduced a new recipe by replacing TOPO with a mixture of ODE and oleic acid.
Wong said the CBEN research team, which included CBEN Director Vicki Colvin, professor of chemistry, and Nikos Mantzaris, assistant professor of chemical and biomolecular engineering and of bioengineering, had some initial doubts about whether heat-transfer fluids could be substituted for ODE.
"They were cheap and they didn't break down at high temperatures, but no one uses these compounds for chemical reactions," said Wong. In addition to finding that other quantum dot nanostructures could be made in heat- transfer fluids, the team concluded that any solvent could be used to replace ODE. Thanks to a mathematical modeling approach developed by Mantzaris, the team now has a method for predicting the particle size and growth behavior of quantum dots based on only three physical properties of a given solvent: viscosity, surface free energy and solubility of bulk cadmium selenide powder.
Source: Rice University