April 3, 2015 report
Crystal studies reveal malaria's weak spots
(Phys.org)—The World Health Organization's 2014 report on worldwide malaria cases showed that while there has been a significant decrease in the incidence of malaria, overall, there were still 198 million cases reported in 2013, with an estimated 584,000 deaths. Most deaths are due to the more lethal form of malaria that is derived from P. falciparum. While artemisinin combination therapies have been effective in fighting malaria, five countries have reported artemisinin resistance, with the area along the Cambodia-Thailand border reporting resistance to almost all available antimalarial drugs.
To find new and better drugs to combat malaria, scientists need to understand the mechanism by which toxic hematin forms in the body and how drugs like the classic antimalarial drug, chloroquine, serve to inhibit this process. To this end, a group from the University of Houston has elucidated, for the first time, the mechanism by which hematin crystals form as well as how chloroquine inhibits this process. Their work is reported in the Proceedings of the National Academy of Sciences.
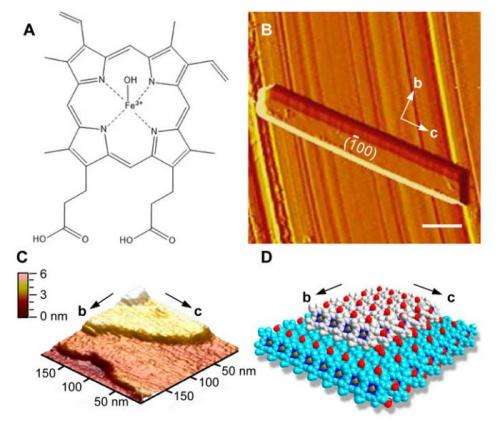
When the malarial parasite consumes hemoglobin in red blood cells, it releases toxic hematin which is then converted into non-toxic hemazoin crystals. As long as hemazoin crystals are formed, the parasite survives. Even though hemozoin crystals were identified as early as the late nineteenth century, scientists are still unsure of the fundamental mechanisms by which hemozoin crystals are grown within the parasite. Additionally, while there are theories as to how antidotes, like chloroquine, act to inhibit crystallization, the mechanism has remained unclear. Katy N. Olafson, Megan A. Ketchum, Jeffrey D. Rimer, and Peter G. Vekilov from the Department of Chemical and Biomolecular Engineering and the Department of Chemistry at the University of Houston sought to investigate this process using model systems and in situ atomic force microscopy (AFM).
Olafson et al. began by looking at whether hemazoin crystals would form in an aqueous or organic environment. There has been some debate over whether the parasite's digestive vacuole, where the crystals form, is largely aqueous or organic. To understand this crystallization process better, Olafson et al. used β-hematin, a synthetic analog of hemazoin, and tested it under biologically analogous conditions.
AFM studies showed that citric acid buffer-saturated n-octanol (CBSO) was the best solvent to promote crystal growth, indicating that both the aqueous and lipid environments are likely necessary for crystal formation. Additionally, chloroquine was found to be less soluble in CBSO compared to an aqueous solvent, but still more soluble than hematin, which is important for chloroquine's inhibitory activity.
The next step was to evaluate the crystallization mechanism of β-hematin in CBSO. Olafson et al. performed the first time-resolved in situ AFM study of hematin crystal growth. Their studies showed that β-hematin has a similar morphology to hemazoin crystals. Hematin seems to follow classical layer-by-layer crystal growth, and new layers nucleate and grow by the attachment of solute molecules to each new layer. Their evaluation of crystal growth rate and concentration suggest that hemazoin's in vivo concentration is likely very close to 0.22 mM.
These studies indicate that hemazoin crystals must form rapidly after hematin is released. According to the authors, even a moderate delay in crystallization may induce a significant accumulation of toxic hematin, which would serve to remove the parasite from the host. They identified four classes of sites on the hematin crystals that were key sites for crystal growth and may serve as potential binding sites for new malaria therapies.
Finally, they investigated the mechanism by which chloroquine acts. Chloroquine, indeed, binds to one of these four sites on the hematin crystal surface, inhibiting further crystal growth. AFM studies show that chloroquine will preferentially adsorb to the crystal surface. Crystal formation was found to be highly sensitive to chloroquine concentration, suggesting that malarial resistance to chloroquine may be due to a mechanism that controls its concentration.
This research has elucidated several key features of malaria that can be exploited for finding new therapies. According to Dr. Peter Vekilov, these findings will help in the search for new antimalarials in several ways, "First, we show that tests of the inhibitory activity of potential new antimalarials should be carried out in organic and not in purely aqueous solvents. Second, our findings suggest that antimalarials of the quinoline class, the most populous class of such drugs, act by binding to specific sites on the hematin crystal surfaces and not by sequestering hematin molecules dissolved in the crystallization medium. Third, we provide atomic resolution data on the structure of the binding sites available for drug association that may serve as the basis for detailed molecular modelling of the inhibition events of potential drug molecules."
Dr. Jeffrey Rimer, when asked about the implications of their research, said, "We envision that hematin crystallization assays can be used as an initial step in drug screening to more efficiently identify lead candidates. Moreover, studies of hematin growth inhibition can elucidate fundamental aspects of drug-crystal molecular recognition towards the rational design of the next generation anti-malarial drugs."
More information: "Mechanisms of hematin crystallization and inhibition by the antimalarial drug chloroquine" PNAS, DOI: 10.1073/pnas.1501023112
Abstract
Hematin crystallization is the primary mechanism of heme detoxification in malaria parasites and the target of the quinoline class of antimalarials. Despite numerous studies of malaria pathophysiology, fundamental questions regarding hematin growth and inhibition remain. Among them are the identity of the crystallization medium in vivo, aqueous or organic; the mechanism of crystallization, classical or nonclassical; and whether quinoline antimalarials inhibit crystallization by sequestering hematin in the solution, or by blocking surface sites crucial for growth. Here we use time-resolved in situ atomic force microscopy (AFM) and show that the lipid subphase in the parasite may be a preferred growth medium. We provide, to our knowledge, the first evidence of the molecular mechanisms of hematin crystallization and inhibition by chloroquine, a common quinoline antimalarial drug. AFM observations demonstrate that crystallization strictly follows a classical mechanism wherein new crystal layers are generated by 2D nucleation and grow by the attachment of solute molecules. We identify four classes of surface sites available for binding of potential drugs and propose respective mechanisms of drug action. Further studies reveal that chloroquine inhibits hematin crystallization by binding to molecularly flat {100} surfaces. A 2-μM concentration of chloroquine fully arrests layer generation and step advancement, which is ∼104× less than hematin's physiological concentration. Our results suggest that adsorption at specific growth sites may be a general mode of hemozoin growth inhibition for the quinoline antimalarials. Because the atomic structures of the identified sites are known, this insight could advance the future design and/or optimization of new antimalarials.
Journal information: Proceedings of the National Academy of Sciences
© 2015 Phys.org