Global bonds boost chemists' pace of research and discovery

A pair of chemistry graduate students, from Emory and Nagoya University in Japan, combined forces to demonstrate how a newer, more efficient strategy can be applied to synthesize natural compounds that hold potential medicinal benefits.
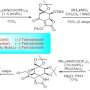
The Journal of the American Chemical Society (JACS) published their findings, showing how C-H functionalization speeds up synthesis of two promising marine alkaloids from a sea sponge, known as dictyodendrin A and F.
"We were able to cut the number of steps needed to synthesize these products nearly in half, compared to previous, more traditional methods," says Kathryn (Katie) Chepiga, an Emory PhD student of organic chemistry. "The ability to more efficiently synthesize them greatly improves the chances that they will be produced on a larger scale so that more can be learned about their biological properties and potential benefits."
Previous research has found that dictyodendrin A inhibits telomerase, suggesting its potential for cancer chemotherapy. And dictyodendrin F inhibits an amyloid-cleaving enzyme, hinting at its potential to treat Alzheimer's disease.
Chepiga shares lead authorship of the JACS paper with Atsushi Yamaguchi, a graduate student from Nagoya University. Their professors and mentors are co-authors of the paper, including Huw Davies at Emory and Kenichiro Itami and Junichiro Yamaguchi from Nagoya.
Both graduate students spent time working on the problem at one another's universities through an exchange program of the National Science Foundation's Center for Selective C-H Functionalization (CCHF).
"This paper shows the power of the global network the CCHF has developed," says Davies, director of the center, which is based at Emory. "We hope this work serves as a model for others to emulate and to expand upon – both the new methods of doing chemical synthesis and the new ways for organic chemists to combine their expertise and collaborate, rather than compete."
The graduate students completed the synthesis of the two products over the course of one year.
"It's common for a total synthesis project to take four to six years," Davies says. "It's amazing that they achieved it in such a short time. Emory had one area of expertise needed to complete the project, and the University of Nagoya had the other area. Katie and Atsushi bridged cultures and continents and brought these two areas together."
The project began in the fall of 2013, when Atsushi Yamaguchi traveled to Atlanta to spend three months working in the Davies lab. He brought with him an idea from the Itami lab in Nagoya – to apply C-H functionalization methods to synthesize dictyodendrins.

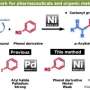
Traditionally, organic chemistry has focused on the division between reactive, or functional, molecular bonds and the inert, or non-functional bonds carbon-carbon (C-C) and carbon-hydrogen (C-H). The inert bonds provide a strong, stable scaffold for performing chemical synthesis on the reactive groups.
C-H functionalization flips this model on its head: It bypasses the reactive groups and does synthesis at the inert C-H sites.
The CCHF is at the forefront of this major paradigm shift in organic chemistry. It brings together scientists from leading research universities across the United States, Asia and Europe – as well as from private industry – with the aim of making organic synthesis faster, simpler and greener.
At the same time, the center is preparing students for a new era of collaborative chemistry on a global scale. Undergraduates, graduate students and post-doctoral fellows can participate in national and international exchanges, learning the techniques of other labs while bringing in new ideas of their own.
When Atsushi Yamaguchi arrived at Emory, he hit the ground running, Davies recalls. "The culture of our lab is very different from the way they work in Japan, but Atsushi just jumped right in and embraced it. He was very focused and extremely determined to learn all that he could and to make his project work."
Chepiga shares a similar determination, as well as the desire to gain varied experiences. She entered Emory's graduate program in chemistry in 2010, drawn by the exchange opportunities offered by the CCHF.
The center began with a network of top research universities across the United States when it launched in 2009. Since then, it has expanded through the NSF program Science Across Virtual Institutes (SAVI) to also include organic chemistry labs and research centers in Japan, Korea, England and Germany.
"In organic chemistry, you might spend your whole PhD program just learning the techniques and expertise of one lab and one professor," Chepiga says. "When I heard how the center was changing that concept, I wanted to be a part of it. I'm gaining a range of expertise and learning how to adapt to different lab settings. And I have a much bigger network of professors and students to bounce ideas off of when I run into a problem. It never feels like there is a dull moment in a project because we can come at it from so many different perspectives."
Last spring, Chepiga traveled to Nagoya University for three months to help Atsushi Yamaguchi complete the synthesis project. "I loved the cultural experience and working in a new environment," she says. "I found the members of the Itami lab to be incredibly friendly and helpful."
The challenge facing the two graduate students was to perform controlled, sequential functionalization of four C-H bonds on a pyrrole core – a basic, organic unit common in many medicinal compounds.
The Itami lab is specialized in C-H arylation, a process that converts a C-H bond into an elaborate structure needed for the synthesis. The Davies lab is specialized in using rhodium catalysts to directly insert a carbine fragment into C-H bonds, another critical step.
"We definitely struggled at times," says Chepiga of the problems involved. She asked for an additional month in Japan to see the project through.
While her work at the Davies lab has focused on catalyst development and applications, the exchange project required Chepiga to learn new techniques for synthesis and for analyzing the products of small-scale reactions.
The hard work paid off when they achieved results and publication by JACS.
"It is a great feeling of accomplishment," Chepiga says. "We're hoping that other researchers will want to explore the potential therapeutic benefits of dictyodendrins A and F, now that the synthesis is more practical. And we also hope that our synthesis methods can be applied more broadly to many other compounds with interesting biological properties."
Combining the expertise of different labs not only boosted the pace of discovery, it also speeded up Chepiga's academic career. She is on track to finish her PhD program within a few months, for a total of just 4.5 years, and she has already secured a post-doctoral fellowship in Germany.
Journal information: Journal of the American Chemical Society
Provided by Emory University