July 4, 2014 report
Researchers find unfolded-protein-response can both activate and degrade cell death receptor 5 protein
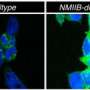
(Phys.org) —A team of researchers with members from several facilities in California and from one in Australia has found evidence that an unfolded-protein-response can both activate and degrade the death receptor 5 protein (DR5). As the team describes in their paper published in the journal Science, their findings suggest that the same mechanism responsible for cell death due to stress, also plays a role in initially blocking cell suicide (apoptosis).
Protein folding by the endoplasmic reticulum (ER) is a critical part of cell function, if it's disrupted, ER stress results which leads to a variety of pathological processes in many diseases. For this reason, researchers the world over have been conducting research into the mechanism involved as part of a mission to better understand the process, which would hopefully lead to the prevention of undesirable stress and cell death, and cures for the diseases that result. In this new effort, the researchers report that they believe they have found a cellular stress pathway that illuminates the link between apoptosis and an unfolded protein response. The results of their research suggest that when protein folding doesn't happen correctly, unfolded proteins build up in the cell causing stress. When that happens, an un-folded protein response is activated—unfolded proteins are degraded, translation is stopped and the mechanism that causes protein folding is bolstered. If that doesn't work, the team reports, the same process can set off the process of apoptosis.
It's a matter of degree, the researchers suggest, a little stress appears to be tolerable—it's only when the stress carries on for a certain length of time that apoptosis occurs by activation of the DR5 protein.
The results announced by the team have already been met with mixed results from others studying the same process—some suggest that the significance of the process is still not demonstrated, while others suggest the findings by the researchers have provided a simple explanation for a very complex process. In any case, most agree that more research needs to be conducted, both to determine the significance of what the researchers have found and whether it might lead to progress treating diseases.
More information: Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis, Science 4 July 2014: Vol. 345 no. 6192 pp. 98-101 . DOI: 10.1126/science.1254312
ABSTRACT
Protein folding by the endoplasmic reticulum (ER) is physiologically critical; its disruption causes ER stress and augments disease. ER stress activates the unfolded protein response (UPR) to restore homeostasis. If stress persists, the UPR induces apoptotic cell death, but the mechanisms remain elusive. Here, we report that unmitigated ER stress promoted apoptosis through cell-autonomous, UPR-controlled activation of death receptor 5 (DR5). ER stressors induced DR5 transcription via the UPR mediator CHOP; however, the UPR sensor IRE1α transiently catalyzed DR5 mRNA decay, which allowed time for adaptation. Persistent ER stress built up intracellular DR5 protein, driving ligand-independent DR5 activation and apoptosis engagement via caspase-8. Thus, DR5 integrates opposing UPR signals to couple ER stress and apoptotic cell fate.
Journal information: Science
© 2014 Phys.org