Theoretical chemists guide experimentalists in search for more efficient production of hydrogen
(Phys.org) —Hydrogen can be used to power cars with zero pollution because it burns with oxygen in the air, producing nothing but water, which is harmless. Hydrogen is also widely used to produce other chemicals. For example, ammonia, which is the fertilizer that everyone uses to raise the crops, is made with hydrogen and nitrogen.
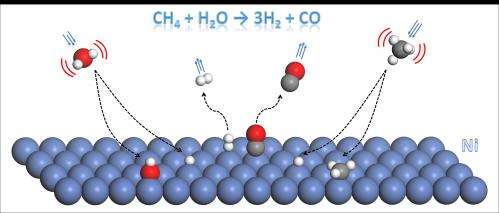
Currently, the main route for producing hydrogen on an industrial scale is a process called steam reforming, in which water vapor and methane react at high temperatures on nickel catalysts to produce hydrogen and carbon dioxide. Steam reforming is energy intensive, as the process requires a high operational temperature.
In search for a more efficient way to producing hydrogen, researchers have been experimenting with lasers to excite the reactants (methane and water), instead of heating them. A breakthrough recently published on the prestigious research journal Science by Chemistry Professor Hua Guo and his collaborators at UNM and Ecole Polytechnique Fédérale de Lausanne (EPFL) has shown that exciting the stretching vibration of water can significantly accelerate its dissociation on Ni much more effectively than heating the molecule.
Funded by the National Science Foundation, Guo has been toying with the idea of pre-treating water by exciting its vibration and letting it stretch a little more. The researchers wanted to see whether that was equivalent to heating the whole thing up.

"If you put energy into the right molecular vibrational mode, let's say the asymmetric stretch, you may push the reaction over the barrier more efficiently." Guo said. "If you heat it up, that always works, but that costs a lot of energy. We wondered if one could use a laser for example to put energy into a vibrational mode of the molecule, and that mode would help you overcome the barrier. It looks like it's not only possible, it's actually very effective."
Two years ago, Guo, and his postdoc Bin Jiang predicted vibrational enhancement of the dissociation of water based on their theoretical research. "It was one of those cases where we have made a theoretical prediction and it was confirmed by experimentalists." he said. "That was really cool."
Catalysis is a hugely important research area, as it lays the foundation for future technologies that will impact almost every aspects of our society. The work carried out at UNM demonstrated that it has become possible to accurately predict complicated chemical processes such as catalysis using theoretical modeling and large scale computation. Many such studies are currently been performed at UNM's Center for Advanced Research Computing.
Journal information: Science
Provided by University of New Mexico


















