April 22, 2013 report
Simulation shows it's possible to move H2O@C60 using electrical charge

Bob Yirka
news contributor
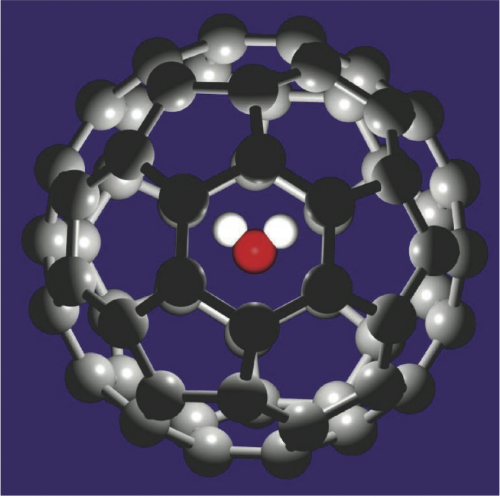
(Phys.org) —Researchers Baoxing Xu and Xi Chen, working at Columbia University, have created a computer simulation that shows it's possible to manipulate the movement of a 60-atom fullerene, with a water molecule trapped inside of it, using an electrical charge. They describe their simulation and results in their paper published in Physical Review Letters.
Two years ago, Japanese researchers Kei Kurotobi and Yasujiro Murata figured out a way to imbed a water molecule in a 60-atom fullerene (buckeyball)—they slit it open, inserted a single water molecule, then sealed it back up again, effectively trapping the water molecule inside—they called it H2O@C60. In this new effort, the researchers created a computer simulation which they claim shows what would happen if such a molecule were placed inside a nanotube and subjected to an electrical charge. Their efforts show, they say, that it would cause the fullerene (and water molecule) to move, in this case through a channel.
David Lindley, in an article for the American Physical Society site Physics, says that the simulation the two researchers created takes into account all of the known properties of H2O@C60 and notes that the simulation treats the molecule as a single entity.
After embedding the water molecule inside the fullerene, the researchers simulated putting the new structure inside of a carbon nanotube, essentially creating a channel to allow for movement of the fullerene along with its water molecule cargo. They then applied an electrical charge parallel to the nanotube. Doing so, the researchers found, caused the fullerene to move within the channel (and the water molecule inside to spin), carrying its cargo with it. Normally, applying an electrical charge to water molecules does not cause them to move (because they are neutrally charged)—instead a thermal driven motion known as libration occurs.
In the simulation however, embedding a water molecule in a fullerene allows it to be driven through a channel using electric current, opening up the possibility of creating fullerenes that carry other chemicals through nanotubes—a process that could prove useful for applications such as delivering therapeutic drugs to ailing body parts, for example.
Interestingly, the researchers found that if the charge was increased to 0.065 volts per angstrom, the direction of movement in the channel was reversed, though they can't explain why.
Written for you by our author Bob Yirka—this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a donation (especially monthly). You'll get an ad-free account as a thank-you.
More information: Electrical-Driven Transport of Endohedral Fullerene Encapsulating a Single Water Molecule, Phys. Rev. Lett. 110, 156103 (2013) prl.aps.org/abstract/PRL/v110/i15/e156103
Abstract
Encapsulating a single water molecule inside an endohedral fullerene provides an opportunity for manipulating the H2O@C60 through the encapsulated polar H2O molecule. Using molecular dynamic simulations, we propose a strategy of electrical-driven transport of H2O@C60 inside a channel, underpinned by the unique behavior of a water molecule free from a hydrogen-bonding environment. When an external electrical field is applied along the channel's axial direction, steady-state transport of H2O@C60 can be reached. The transport direction and rate depend on the applied electrical intensity as well as the polar orientation of the encapsulated H2O molecule.
Journal information: Physical Review Letters
© 2013 Phys.org