Three-dimensional fiber scaffold promotes large-scale stem cell proliferation and differentiation
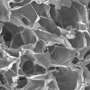
Thanks to the ability of pluripotent stem cells to self-renew and differentiate into a wide variety of specialized cell types, they are expected to revolutionize the treatment of illnesses such as type I diabetes and Parkinson's disease. Before this becomes a reality, however, scientists must develop culture systems to mass-produce these cells. To overcome the limitations of previous single-layer-substrate systems, a research team in Singapore has developed three-dimensional scaffolds that stimulate stem cell proliferation and differentiation under defined chemical conditions. Importantly, the system can be scaled up. The scaffolds consist of microscopic fibers obtained by weaving together polymer strands bearing opposite charges.
Hongfang Lu and Andrew Wan from the A*STAR Institute of Bioengineering and Nanotechnology led the research. Wan notes that the fiber-based scaffold not only avoids the need to consume large quantities of key growth factors, but it would also shield the cells from the shear stresses generated in large-scale bioreactors.
To manufacture the scaffold, the researchers opted for a positively charged biopolymer called chitin, which they extracted from crab shell, and a negatively charged polymer called sodium alginate. After depositing one droplet of each of these water-soluble polymers onto a sterile substrate, they brought the droplet interfaces into contact using forceps; this formed a chitin–alginate complex. Held together by intermolecular electrostatic interactions, the complex extended into a continuous fiber. The team reeled the fiber onto a holder to complete the three-dimensional system.
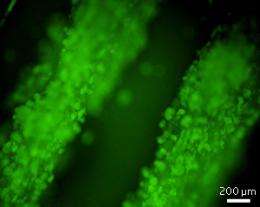
By suspending the stem cells in the alginate solution, Lu, Wan and co-workers incorporated the cells into the scaffold during fiber formation, resulting in a network of uniformly distributed cells (see image). Preliminary tests showed that when the researchers destroyed the scaffold with enzymes, they could recover a high number of the cells.
Lu explains that their system provided a 'micro-environment' in which cells could grow in aggregates. When sub-cultured over many generations, the encapsulated stem cells remained pluripotent and did not undergo any genetic mutations. Moreover, the cells displayed excellent viability when frozen in the fiber for storage; in addition, they could either self-renew or differentiate, depending on the media available to them. "The small dimensions of the fibers are useful because they allow nutrients and growth factors to efficiently diffuse towards the cells within the scaffold," she adds.
The team is now planning to exploit their approach to produce transplantable tissue for cell-based therapy. "Our system allows us to generate large numbers of cells for tissue-engineering applications," says Wan.
More information
Lu, H. F., Narayanan, K., Lim, S.-X., Gao, S., Leong, M. F. & Wan, A. C. A. A 3D microfibrous scaffold for long-term human pluripotent stem cell self-renewal under chemically defined conditions. Biomaterials 33, 2419–2430 (2012): article