Gone fishing: Researchers' imaging technique trolls in quiet cellular seas
Experienced anglers know that choppy waters make for difficult fishing, so they try not to rock the boat. Thanks to a new microscopy technique, cell biology researchers can heed that same advice.
University of Illinois researchers developed a method they call "trolling AFM," which allows them to study soft biological samples in liquid with high resolution and high quality. Led by mechanical science and engineering professor Min-Feng Yu, the group published its findings in the journal Nanotechnology.
"We developed a highly sensitive method for high-resolution imaging of soft biological samples, such as living cells, in their physiological condition," said Majid Minary, a recent graduate of Yu's group and first author of the paper. Minary now is a professor at the University of Texas-Dallas. "We improved the quality factor of common atomic force microscopy imaging methods by two orders of magnitude," Minary said.
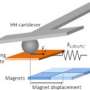
The widely used atomic force microscope provides images of tiny structures with high resolution at the atomic scale. The AFM has a sharp probe at the end of an arm, called a cantilever. The tip of the probe skims the surface of a sample to measure mechanical, electrical or chemical properties.
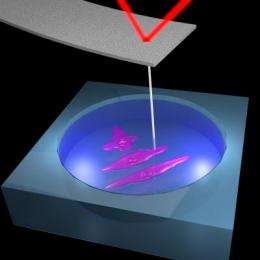
When scientists want to study cells, tissue or other live biological materials, the samples must be submerged in a liquid to keep them alive. This poses difficulties for atomic force microscopy, because the cantilever has to be submerged as well.
Cells and tissues are so soft that if the AFM probe were simply dragged across the surface, it would damage or displace the sample instead of reading it. Therefore, scientists have to operate the AFM in oscillation mode – with the probe gently tapping along the sample and detecting resistance.
But oscillation in liquid brings a tide of complications in its wake.
Oscillating a relatively large structure, such as an AFM cantilever, through liquid also causes the liquid to surge up and down with the oscillation, like waves in a tidal pool, causing even more drag.
"There's a huge amount of hydrodynamic drag associated with operating a such a big cantilever, compared to the resolution you're trying to approach," said Yu, "so it causes lots of disturbance, recorded as noise, which overwhelms all the actual data you're trying to get from the sample."
The high noise level requires the probe to tap harder to find a signal. This means the tip deforms a cell as the probe presses down, and only large, stiff structural elements such as the nucleus are visible, rendering AFM unable to resolve the membrane's structure, properties and contours with high resolution.
Yu's group devised a solution to the problem by allowing the cantilever to oscillate in air above the liquid while the sample is still submerged. They attached a thin, long nanoneedle – a structure the group developed previously – to the end of the probe, effectively extending the tip.
"We call it 'trolling mode' AFM, as in fishing where a part of the fishing line is immersed in water and the other part above," Yu said.
While AFM of soft tissues with a submerged probe is like trying to club fish with a large paddle in a wave pool, the new arrangement is like trolling a fishing line in a calm pond. The nanoneedle displaces very little of the liquid and causes very little drag, yet is very responsive, so that the cantilever can oscillate very gently with very small amplitude.
"Once you remove the noise, all the information you're getting is from the sample, instead of from the interaction between the tip and the liquid," Yu said.
Using trolling AFM, the group gained high-resolution topographical images of human cells.
"We can tap with such small force that we can reveal the regional contours of the membrane," said Ning Wang, a professor of mechanical science and engineering and a co-author of the paper. "Not only that, more importantly, we get the viscoelastic map. We put a little bit of force on it, and see how viscoelastic it is."
Thanks to the minimal disturbance, trolling AFM also can operate at high frequency, which could allow researchers to study the dynamics of cellular structures that previously were not detectable.
Next, the researchers want to expand the utility of this instrument with additional dynamic measurement capability. The team also will work with biologists to identify issues relating to cell membrane and refine trolling AFM to resolve structures in the membrane.
More information: The paper, "Intrinsically High-Q Dynamic AFM Imaging in Liquid With a Significantly Extended Needle Tip," is available online at iopscience.iop.org/0957-4484/23/23/235704/
Journal information: Nanotechnology
Provided by University of Illinois at Urbana-Champaign