Hydrogen fuel for thought: Researchers find metallacarboranes may meet DOE storage goals
(PhysOrg.com) -- New research by Rice University scientists suggests that a class of material known as metallacarborane could store hydrogen at or better than benchmarks set by the United States Department of Energy (DOE) Hydrogen Program for 2015.
The work could receive wide attention as hydrogen comes into play as a fuel of the future for cars, in fuel cells and by industry.
The new study by Rice theoretical physicist Boris Yakobson and his colleagues, which appears in the online Journal of the American Chemical Society, taps the power of transition metals scandium and titanium to hold a load of hydrogen molecules -- but not so tightly that they can't be extracted.
A matrix made of metallacarboranes would theoretically hold up to 8.8 percent of its weight in hydrogen atoms, which would at least meet and perhaps surpass DOE milestones issued a year ago for cars that would run on hydrogen fuel.
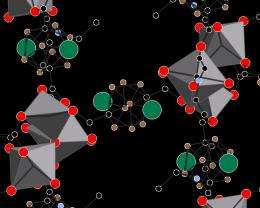
Yakobson, a professor in mechanical engineering and materials science and of chemistry at Rice, said inspiration for the new study came from the development of metallacarboranes, now well-known molecules that combine boron, carbon and metal atoms in a cage-like structure.
"A single metal atom can bind multiple hydrogen molecules," Yakobson said, "but metals also tend to aggregate. Without something to hold them, they clump into a blob and are useless."
Abhishek Singh, lead author of the study, a former postdoctoral researcher for Yakobson and now an assistant professor at the Indian Institute of Science in Bangalore, India, calculated that boron clusters would grip the titanium and scandium, which would in turn bind hydrogen. "The metals fit like a gem in a setting, so they don't aggregate," Yakobson said. Carbon would link the clusters to form a matrix called a metal organic framework (MOF), which would act like a sponge for hydrogen.
Investigation of various transition metals showed scandium and titanium to have the highest rate of adsorption (the adhesion of transient molecules -- like hydrogen -- to a surface). Both demonstrate an affinity for "Kubas" interaction, a trading of electrons that can bind atoms to one another in certain circumstances. "Kubas is a special interaction that you often see mentioned in hydrogen research, because it gives exactly the right binding strength," Yakobson said.
"If you remember basic chemistry, you know that covalent bonds are very strong. You can bind hydrogen, but you cannot take it out," he said. "And on the other extreme is weak physisorption. The molecules don't form chemical bonds. They're just exhibiting a weak attraction through the van der Waals force.
"Kubas interaction is in the middle and gives the right kind of binding so hydrogen can be stored and, if you change conditions -- heat it up a little or reduce pressure -- it can be taken out. You want the framework to be like a fuel tank."
Kubas allows for reversible storage of hydrogen in ambient conditions -- ranging from well above to well below room temperature -- and that would make metallacarborane materials highly attractive for everyday use, Yakobson said. Physisorption of hydrogen by the carbon matrix, already demonstrated, would also occur at a much lower percentage, which would be a bit of a bonus, he said.
Other studies have demonstrated how to make carborane-based MOFs. "That means they can already make three-dimensional frameworks of material that are still accessible to gas. This is very encouraging to us," Yakobson said. "There are many papers where people analyze a cluster and say, 'Oh, this will also absorb a hydrogen,' but that's not useful. One cluster is nothing.
"But if chemists can synthesize this particular framework with metallacarborane as an element, this may become a reality."
More information: pubs.acs.org/doi/abs/10.1021/ja104544s
Provided by Rice University


















