Move to the red! Design and synthesis of rigid fluorophores
(PhysOrg.com) -- Stable dyes with sharp absorption and fluorescence emission bands in the red or NIR region of the spectrum, combined with high molar absorption coefficients and high fluorescence quantum yields, may find extensive use in many different fields, such as optical engineering, analytical chemistry, biological in vivo imaging and sensing applications, and materials science.
In Chemistry -- An Asian Journal, Wim Dehaen and co-workers, based at Katholieke Universiteit Leuven (Belgium), Lanzhou University (China), and the Université de Mons (Belgium) describe the preparation of difluoroboron dipyrromethene (BODIPY)-based dyes with increasing conformational rigidity that have absorption in the visible region of the spectrum.
Although a substantial number of BODIPY dye analogues have been developed, primarily through extended conjugation using aryl substituents, the substituted BODIPY dyes show red shifts of over 100 nm but have only low to moderate fluorescence quantum yields owing to non-radiative decay arising from the non-rigid nature of compounds. Alkenyl, and more-recently alkynyl substituents afforded large red shifts, but the rigidity of the triple bond generally results in higher quantum yields. Functionalization of the aromatic rings attached to the BODIPY core with heterocyclic rings even led to near infrared (NIR) emission; however, these dyes require lengthy multi-step syntheses of the fused-ring pyrrole starting materials and are restricted in scope to symmetrical frameworks.
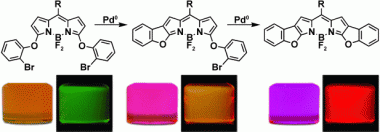
Two BODIPY dyes were synthesized from a conformationally unconstrained indacene using simple palladium catalysis. These dyes showed restricted rotation of their phenoxy moieties, and thus absorb and fluoresce more intensely at longer wavelengths relative to their unrestricted analogues. Furthermore, reduction of the conformational flexibility in these dyes led to significantly higher fluorescence quantum yields. Quantum chemical calculations were also performed which showed that the increase in conformational constraint led to larger spectroscopic shifts. X-ray diffraction analysis showed a progressive increase in the extended planarity of the chromophore in line with increasing conformational rigidity, which explained the larger red shifts in the absorption and emission spectra.
This practically simple design strategy provides promise for the rapid development of new BODIPY-based dyes with increasing structural rigidity. Furthermore, the development of novel dyes with extended planarity is expected to afford higher quantum yields and more-substantial bathochromic shifts into the NIR region, which may find interesting application in a wide variety of fields.
More information:
Wim Dehaen, Synthesis, Spectroscopy, Crystal Structure Determination, and Quantum Chemical Calculations of BODIPY Dyes with Increasing Conformational Restriction and Concomitant Red-Shifted Visible Absorption and Fluorescence Spectraб
Chemistry - An Asian Journal 2010, 5, No. 9, 2016-2026, dx.doi.org/10.1002/asia.201000248
Provided by Wiley